Differential expression of microRNA, miR-150 and enhancer of zeste homolog 2 (EZH2) in peripheral blood cells as early prognostic markers of severe forms of dengue
- PMID: 31954402
- PMCID: PMC6969970
- DOI: 10.1186/s12929-020-0620-z
Differential expression of microRNA, miR-150 and enhancer of zeste homolog 2 (EZH2) in peripheral blood cells as early prognostic markers of severe forms of dengue
Abstract
Background: Dengue presents a wide clinical spectrum. Most patients recover following a self-limiting non-severe clinical course. A small proportion of patients progress to severe disease, mostly characterized by plasma leakage with or without hemorrhage. Early symptoms of severe dengue (SD) are similar to those of non-severe dengue fever (DF). Severe symptoms manifest after 3-5 days of fever, which can be life threatening due to lack of proper medications and inability to distinguish severe cases during the early stages. Early prediction of SD in patients with no warning signs who may later develop severe infection is very important for proper disease management to alleviate related complications and mortality. microRNA are small non-coding RNA molecules that regulate post-transcriptional gene expression. Due to the remarkable stability and the role of microRNA in gene expression, altered expression of microRNA was evaluated to explore clinically relevant prognostic markers of severe dengue.
Methods: The relative expression of microRNA hsa-let-7e (let-7e), hsa-miR-30b-5p (miR-30b), hsa-miR-30e-3p (miR-30e), hsa-miR-33a (miR-33a), and hsa-miR-150-5p (miR-150) and several putative target genes in peripheral blood cells (PBC) collected from 20 DF and 20 SD positive patients within 4 days from fever onset was evaluated by quantitative reverse transcription PCR (qRT-PCR).
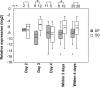
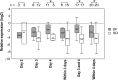
Results: miR-150 showed significant (P < 0.01) up regulation in PBC of SD patients compared to DF patients during the acute phase of infection. Expression of enhancer of zeste homolog 2 (EZH2) was significantly (P < 0.01) down regulated indicating that genes involved in epigenetic regulation are also differentially expressed in SD patients during the early stage of infection.
Conclusions: Differential expression of microRNA miR-150 and the putative target gene EZH2 may serve as reliable biomarkers of disease severity during early stages of dengue infection.
Keywords: Acute dengue biomarkers; Dengue; Severe dengue; microRNA.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
MicroRNA profiling reveals dysregulated microRNAs and their target gene regulatory networks in cemento-ossifying fibroma.J Oral Pathol Med. 2018 Jan;47(1):78-85. doi: 10.1111/jop.12650. Epub 2017 Nov 1. J Oral Pathol Med. 2018. PMID: 29032608
-
Let-7e inhibits TNF-α expression by targeting the methyl transferase EZH2 in DENV2-infected THP-1 cells.J Cell Physiol. 2018 Nov;233(11):8605-8616. doi: 10.1002/jcp.26576. Epub 2018 May 16. J Cell Physiol. 2018. PMID: 29768655
-
Enhancer of zeste homolog 2 participates in the process of atherosclerosis by modulating microRNA-139-5p methylation and signal transducer and activator of transcription 1 expression.IUBMB Life. 2021 Jan;73(1):238-251. doi: 10.1002/iub.2423. Epub 2020 Dec 17. IUBMB Life. 2021. PMID: 33331071
-
Risk factors and biomarkers of severe dengue.Curr Opin Virol. 2020 Aug;43:1-8. doi: 10.1016/j.coviro.2020.06.008. Epub 2020 Jul 17. Curr Opin Virol. 2020. PMID: 32688269 Review.
-
Risk and predictive factors for severe dengue infection: A systematic review and meta-analysis.PLoS One. 2022 Apr 15;17(4):e0267186. doi: 10.1371/journal.pone.0267186. eCollection 2022. PLoS One. 2022. PMID: 35427400 Free PMC article.
Cited by
-
Proteomics Analysis of Peripheral Blood Mononuclear Cells from Patients in Early Dengue Infection Reveals Potential Markers of Subsequent Fluid Leakage.Viruses. 2025 May 31;17(6):805. doi: 10.3390/v17060805. Viruses. 2025. PMID: 40573396 Free PMC article.
-
Correlation of miR-150, hsa-let-7e, and miR- 146a and gene expression of IL-6, IL-8, IP-10, and MIP-1β during dengue virus infection.Narra J. 2021 Apr;1(1):e31. doi: 10.52225/narraj.v1i1.31. Epub 2021 Apr 1. Narra J. 2021. PMID: 38449776 Free PMC article.
-
Regulation of Host Innate Immunity by Non-Coding RNAs During Dengue Virus Infection.Front Cell Infect Microbiol. 2020 Nov 30;10:588168. doi: 10.3389/fcimb.2020.588168. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33330133 Free PMC article. Review.
-
Cross Talk between MicroRNAs and Dengue Virus.Am J Trop Med Hyg. 2024 Apr 2;110(5):856-867. doi: 10.4269/ajtmh.23-0546. Print 2024 May 1. Am J Trop Med Hyg. 2024. PMID: 38579704 Free PMC article. Review.
-
Innate Immune Cytokine Profiling and Biomarker Identification for Outcome in Dengue Patients.Front Immunol. 2021 Jul 14;12:677874. doi: 10.3389/fimmu.2021.677874. eCollection 2021. Front Immunol. 2021. PMID: 34335578 Free PMC article.
References
-
- World Health Organization (WHO) and the Special Programme for Research and Tropical Diseases (TDR). Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: WHO; 2009. https://apps.who.int/iris/handle/10665/44188. New Edition: Accessed 02 Sept 2019
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

