mRECIST for HCC: Performance and novel refinements
- PMID: 31954493
- PMCID: PMC12452114
- DOI: 10.1016/j.jhep.2019.09.026
mRECIST for HCC: Performance and novel refinements
Abstract
In 2010, modified RECIST (mRECIST) criteria were proposed as a way of adapting the RECIST criteria to the particularities of hepatocellular carcinoma (HCC). We intended to overcome some limitations of RECIST in measuring tumour shrinkage with local and systemic therapies, and also to refine the assessment of progression that could be misinterpreted with conventional RECIST 1.1, due to clinical events related to the natural progression of chronic liver disease (development of ascites, enlargement of lymph nodes, etc.). mRECIST has served its purpose since being adopted or included in clinical practice guidelines (European, American and Asian) for the management of HCC; it has also been instrumental for assessing response and time-to-event endpoints in several phase II and III investigations. Nowadays, mRECIST has become the standard tool for measurement of radiological endpoints at early/intermediate stages of HCC. At advanced stages, guidelines recommend both methods. mRECIST has been proven to capture higher objective response rates in tumours treated with molecular therapies and those responses have shown to be independently associated with better survival. With the advent of novel treatment approaches (i.e. immunotherapy) and combination therapies there is a need to further refine and clarify some concepts around the performance of mRECIST. Similarly, changes in the landscape of standard of care at advanced stages of the disease are pointing towards progression-free survival as a potential primary endpoint in some phase III investigations, as effective therapies applied beyond progression might mask overall survival results. Strict recommendations for adopting this endpoint have been reported. Overall, we review the performance of mRECIST during the last decade, incorporating novel clarifications and refinements in light of emerging challenges in the study and management of HCC.
Keywords: Endpoints; Liver cancer; Systemic therapies; mRECIST, RECIST, trial design.
Copyright © 2019 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
JML is receiving research support from Bayer HealthCare Pharmaceuticals, Eisai Inc, Bristol-Myers Squibb and Ipsen, and consulting fees from Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Eisai Inc, Celsion Corporation, Eli Lilly, Exelixis, Merck, Ipsen, Glycotest, Navigant, Leerink Swann LLC, Midatech Ltd, Fortress Biotech, Sprink Pharmaceuticals, Nucleix and Can-Fite and Roche. RL has nothing to declare with respect to this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


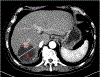
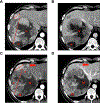
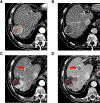

References
-
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. - PubMed
-
- Torre L, Bray F, Siegel R, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. - PubMed
-
- Beaver JA, Howie LJ, Pelosof L, Kim T, Liu J, Goldberg KB, et al. A 25-year experience of US food and drug administration accelerated approval of malignant hematology and oncology drugs and biologics. JAMA Oncol 2018;4:849–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

