Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity
- PMID: 31954621
- PMCID: PMC7322729
- DOI: 10.1016/j.neuron.2019.12.026
Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity
Abstract
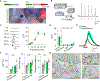
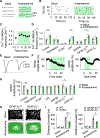
Dopamine is involved in physiological processes like learning and memory, motor control and reward, and pathological conditions such as Parkinson's disease and addiction. In contrast to the extensive studies on neurons, astrocyte involvement in dopaminergic signaling remains largely unknown. Using transgenic mice, optogenetics, and pharmacogenetics, we studied the role of astrocytes on the dopaminergic system. We show that in freely behaving mice, astrocytes in the nucleus accumbens (NAc), a key reward center in the brain, respond with Ca2+ elevations to synaptically released dopamine, a phenomenon enhanced by amphetamine. In brain slices, synaptically released dopamine increases astrocyte Ca2+, stimulates ATP/adenosine release, and depresses excitatory synaptic transmission through activation of presynaptic A1 receptors. Amphetamine depresses neurotransmission through stimulation of astrocytes and the consequent A1 receptor activation. Furthermore, astrocytes modulate the acute behavioral psychomotor effects of amphetamine. Therefore, astrocytes mediate the dopamine- and amphetamine-induced synaptic regulation, revealing a novel cellular pathway in the brain reward system.
Keywords: amphetamine; astrocytes; brain reward system; calcium imaging; dopamine; nucleus accumbens; synaptic transmission.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing financial interests.
Figures

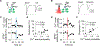


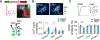
Comment in
-
Dopaminergic Control over the Tripartite Synapse.Neuron. 2020 Mar 18;105(6):954-956. doi: 10.1016/j.neuron.2020.02.031. Neuron. 2020. PMID: 32191856 Free PMC article.
References
-
- Araque A, Parpura V, Sanzgiri RP, and Haydon PG (1999). Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215. - PubMed
-
- Araque A, Carmignoto G, and Haydon PG (2001). Dynamic signaling between astrocytes and neurons. Annu. Rev. Physiol 63, 795–813. - PubMed
-
- Beaulieu JM, and Gainetdinov RR (2011). The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev 63, 182–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

