Tractographic description of major subcortical projection pathways passing the anterior limb of the internal capsule. Corticopetal organization of networks relevant for psychiatric disorders
- PMID: 31954987
- PMCID: PMC6965747
- DOI: 10.1016/j.nicl.2020.102165
Tractographic description of major subcortical projection pathways passing the anterior limb of the internal capsule. Corticopetal organization of networks relevant for psychiatric disorders
Abstract
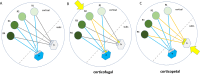
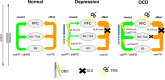
Background: Major depression (MD) and obsessive-compulsive disorder (OCD) are psychiatric diseases with a huge impact on individual well-being. Despite optimal treatment regiments a subgroup of patients remains treatment resistant and stereotactic surgery (stereotactic lesion surgery, SLS or Deep Brain Stimulation, DBS) might be an option. Recent research has described four networks related to MD and OCD (affect, reward, cognitive control, default network) but only on a cortical and the adjacent sub-cortical level. Despite the enormous impact of comparative neuroanatomy, animal science and stereotactic approaches a holistic theory of subcortical and cortical network interactions is elusive. Because of the dominant hierarchical rank of the neocortex, corticofugal approaches have been used to identify connections in subcortical anatomy without anatomical priors and in part confusing results. We here propose a different corticopetal approach by identifying subcortical networks and search for neocortical convergences thereby following the principle of phylogenetic and ontogenetic network development.
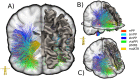
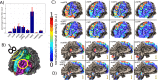
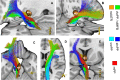
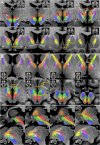
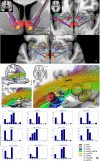
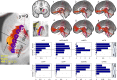
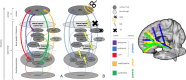
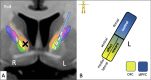
Material and methods: This work used a diffusion tensor imaging data from a normative cohort (Human Connectome Project, HCP; n = 200) to describe eight subcortical fiber projection pathways (PPs) from subthalamic nucleus (STN), substantia nigra (SNR), red nucleus (RN), ventral tegmental area (VTA), ventrolateral thalamus (VLT) and mediodorsal thalamus (MDT) in a normative space (MNI). Subcortical and cortical convergences were described including an assignment of the specific pathways to MD/OCD-related networks. Volumes of activated tissue for different stereotactic stimulation sites and procedures were simulated to understand the role of the distinct networks, with respect to symptoms and treatment of OCD and MD.
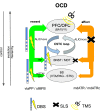
Results: The detailed course of eight subcortical PPs (stnPP, snrPP, rnPP, vlATR, vlATRc, mdATR, mdATRc, vtaPP/slMFB) were described together with their subcortical and cortical convergences. The anterior limb of the internal capsule can be subdivided with respect to network occurrences in ventral-dorsal and medio-lateral gradients. Simulation of stereotactic procedures for OCD and MD showed dominant involvement of mdATR/mdATRc (affect network) and vtaPP/slMFB (reward network).
Discussion: Corticofugal search strategies for the evaluation of stereotactic approaches without anatomical priors often lead to confusing results which do not allow for a clear assignment of a procedure to an involved network. According to our simulation of stereotactic procedures in the treatment of OCD and MD, most of the target regions directly involve the reward (and affect) networks, while side-effects can in part be explained with a co-modulation of the control network.
Conclusion: The here proposed corticopetal approach of a hierarchical description of 8 subcortical PPs with subcortical and cortical convergences represents a new systematics of networks found in all different evolutionary and distinct parts of the human brain.
Keywords: Anterior limb of internal capsule; DBS; Depression; Functional networks; Hyperdirect pathway, Midbrain; MD; Neocortex; OCD; Prefrontal cortex; Projection pathways; Stereotactic lesion surgery.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. - PubMed
-
- Anisman H., Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci. Biobehav. Rev. 2005;29:525–546. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

