Cathelicidin Mediates a Protective Role of Vitamin D in Ulcerative Colitis and Human Colonic Epithelial Cells
- PMID: 31955203
- PMCID: PMC7216768
- DOI: 10.1093/ibd/izz330
Cathelicidin Mediates a Protective Role of Vitamin D in Ulcerative Colitis and Human Colonic Epithelial Cells
Abstract
Background: Vitamin D plays a protective role in ulcerative colitis (UC) patients through unclear mechanisms. Cathelicidin is an antimicrobial peptide induced by 1,25(OH)D2. Our goal was to evaluate the link between cathelicidin and vitamin D-associated clinical outcomes in UC patients, explore vitamin D induction of cathelicidin in human colon cells, and evaluate the effects of intrarectal human cathelicidin on a murine model of colitis.
Methods: Serum and colonic cathelicidin levels were measured in UC patients and correlated with clinical and histologic outcomes. Human colon cells were treated with 1,25(OH)2D and production of cathelicidin and cytokines were quantified. Antimicrobial activity against Escherichia coli from cell culture supernatants was measured. Mice were treated with intrarectal cathelicidin, and its effects on DSS colitis and intestinal microbiota were evaluated.
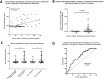
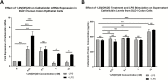
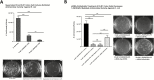
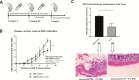
Results: In UC patients, serum 25(OH)D positively correlated with serum and colonic cathelicidin. Higher serum cathelicidin is associated with decreased risk of histologic inflammation and clinical relapse but not independent of 25(OH)D or baseline inflammation. The 1,25(OH)2D treatment of colon cells induced cathelicidin and IL-10, repressed TNF-α, and suppressed Escherichia coli growth. This antimicrobial effect was attenuated with siRNA-cathelicidin transfection. Intrarectal cathelicidin reduced the severity of DSS colitis but did not mitigate the impact of colitis on microbial composition.
Conclusions: Cathelicidin plays a protective role in 25(OH)D-associated UC histologic outcomes and murine colitis. Cathelicidin is induced by vitamin D in human colonic epithelial cells and promotes antimicrobial activity against E. coli. Our study provides insights into the vitamin D-cathelicidin pathway as a potential therapeutic target.
Keywords: cathelicidin; colonic epithelium; cytokines; ulcerative colitis; vitamin D.
© 2020 Crohn’s & Colitis Foundation. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Gubatan J, Chou ND, Nielsen OH, et al. . Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50:1146–1158. - PubMed
-
- Kabbani TA, Koutroubakis IE, Schoen RE, et al. . Association of vitamin D level with clinical status in inflammatory bowel disease: a 5-year longitudinal Study. Am J Gastroenterol. 2016;111:712–719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

