Nutritional ketosis improves exercise metabolism in patients with very long-chain acyl-CoA dehydrogenase deficiency
- PMID: 31955429
- PMCID: PMC7384182
- DOI: 10.1002/jimd.12217
Nutritional ketosis improves exercise metabolism in patients with very long-chain acyl-CoA dehydrogenase deficiency
Abstract
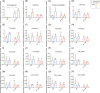
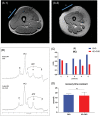
A maladaptive shift from fat to carbohydrate (CHO) oxidation during exercise is thought to underlie myopathy and exercise-induced rhabdomyolysis in patients with fatty acid oxidation (FAO) disorders. We hypothesised that ingestion of a ketone ester (KE) drink prior to exercise could serve as an alternative oxidative substrate supply to boost muscular ATP homeostasis. To establish a rational basis for therapeutic use of KE supplementation in FAO, we tested this hypothesis in patients deficient in Very Long-Chain acyl-CoA Dehydrogenase (VLCAD). Five patients (range 17-45 y; 4 M/1F) patients were included in an investigator-initiated, randomised, blinded, placebo-controlled, 2-way cross-over study. Patients drank either a KE + CHO mix or an isocaloric CHO equivalent and performed 35 minutes upright cycling followed by 10 minutes supine cycling inside a Magnetic Resonance scanner at individual maximal FAO work rate (fatmax; approximately 40% VO2 max). The protocol was repeated after a 1-week interval with the alternate drink. Primary outcome measures were quadriceps phosphocreatine (PCr), Pi and pH dynamics during exercise and recovery assayed by in vivo 31 P-MR spectroscopy. Secondary outcomes included plasma and muscle metabolites and respiratory gas exchange recordings. Ingestion of KE rapidly induced mild ketosis and increased muscle BHB content. During exercise at FATMAX, VLCADD-specific plasma acylcarnitine levels, quadriceps glycolytic intermediate levels and in vivo Pi/PCr ratio were all lower in KE + CHO than CHO. These results provide a rational basis for future clinical trials of synthetic ketone ester supplementation therapy in patients with FAO disorders. Trial registration: ClinicalTrials.gov. Protocol ID: NCT03531554; METC2014.492; ABR51222.042.14.
Keywords: VLCADD; fatty acid oxidation; in vivo 31P MRS; ketone ester; mitochondrial energy transduction; muscle; nutritional ketosis; very long-chain acyl-CoA dehydrogenase.
© 2020 The Authors. Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
The intellectual property and patents covering the uses of ketone bodies and esters are owned by BTG Ltd, The University of Oxford, the NIH, and TΔS Ltd. Should royalties ever accrue from these patents, K.C. and P.J.C. as named inventors may receive a share of royalties as determined by the terms of the respective institutions. K.C. is director of TΔS Ltd, a University of Oxford company with the aim of developing and commercialising products based on the ketone ester. P.J.C. is a former employee of TdeltaS. J.C.B., G.V., S.F., F.H.d.H., R.H.H., L.I., I.L.K., M.L., W.L.v.d.P., M.G.M.d.S.‐v.d.V., A.S.‐K., T.T., R.J.A.W., M.v.W., F.A.W., L.H.v.d.W., R.C.I.W., and J.A.L.J. declare that they have no conflict of interest.
Figures


References
-
- Baruteau J, Sachs P, Broue P, et al. Clinical and biological features at diagnosis in mitochondrial fatty acid beta‐oxidation defects: a French pediatric study of 187 patients. J Inherit Metab Dis. 2013;36:795‐803. - PubMed
-
- Baruteau J, Sachs P, Broue P, et al. Clinical and biological features at diagnosis in mitochondrial fatty acid beta‐oxidation defects: a French pediatric study from 187 patients. Complementary data. J Inherit Metab Dis. 2014;37:137‐139. - PubMed
-
- Bonnet D, Martin D, Pascale De L, et al. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 1999;100:2248‐2253. - PubMed
-
- Spiekerkoetter U. Mitochondrial fatty acid oxidation disorders: clinical presentation of long‐chain fatty acid oxidation defects before and after newborn screening. J Inherit Metab Dis. 2010;33:527‐532. - PubMed
-
- Spiekerkoetter U, Lindner M, Santer R, et al. Management and outcome in 75 individuals with long‐chain fatty acid oxidation defects: results from a workshop. J Inherit Metab Dis. 2009a;32:488‐497. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

