Cryo-EM Reveals Integrin-Mediated TGF-β Activation without Release from Latent TGF-β
- PMID: 31955848
- PMCID: PMC7238552
- DOI: 10.1016/j.cell.2019.12.030
Cryo-EM Reveals Integrin-Mediated TGF-β Activation without Release from Latent TGF-β
Abstract
Integrin αvβ8 binds with exquisite specificity to latent transforming growth factor-β (L-TGF-β). This binding is essential for activating L-TGF-β presented by a variety of cell types. Inhibiting αvβ8-mediated TGF-β activation blocks immunosuppressive regulatory T cell differentiation, which is a potential therapeutic strategy in cancer. Using cryo-electron microscopy, structure-guided mutagenesis, and cell-based assays, we reveal the binding interactions between the entire αvβ8 ectodomain and its intact natural ligand, L-TGF-β, as well as two different inhibitory antibody fragments to understand the structural underpinnings of αvβ8 binding specificity and TGF-β activation. Our studies reveal a mechanism of TGF-β activation where mature TGF-β signals within the confines of L-TGF-β and the release and diffusion of TGF-β are not required. The structural details of this mechanism provide a rational basis for therapeutic strategies to inhibit αvβ8-mediated L-TGF-β activation.
Keywords: GARP; TGF-b signaling; TGF-beta; TGF-beta activation; cryo-electron microscopy; integrin; integrin conformation; structural biology.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests A.C., J.D.M., J.L., J.L.B., Y.C., and S.L.N. are inventors on a patent on anti-v8 antibodies, which have been out-licensed. S.L.N. is on the Scientific Advisory Board of Venn Therapeutics.
Figures
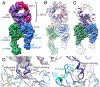




Comment in
-
Targeting TGFβ Signalling in Cancer: Toward Context-Specific Strategies.Trends Cancer. 2020 Jul;6(7):538-540. doi: 10.1016/j.trecan.2020.03.010. Epub 2020 Apr 8. Trends Cancer. 2020. PMID: 32278685
References
-
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, and Rifkin DB (1994). An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 216, 276–284. - PubMed
-
- Annes JP, Munger JS, and Rifkin DB (2003). Making sense of latent TGFbeta activation. J Cell Sci 116, 217–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

