Regulating the Secondary Use of Data for Research: Arguments Against Genetic Exceptionalism
- PMID: 31956328
- PMCID: PMC6951399
- DOI: 10.3389/fgene.2019.01254
Regulating the Secondary Use of Data for Research: Arguments Against Genetic Exceptionalism
Abstract
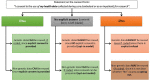
As accessing, collecting, and storing personal information become increasingly easier, the secondary use of data has the potential to make healthcare research more cost and time effective. The widespread reuse of data, however, raises important ethical and policy issues, especially because of the sensitive nature of genetic and health-related information. Regulation is thus crucial to determine the conditions upon which data can be reused. In this respect, the question emerges whether it is appropriate to endorse genetic exceptionalism and grant genetic data an exceptional status with respect to secondary use requirements. Using Swiss law as a case study, it is argued that genetic exceptionalism in secondary use regulation is not justified for three reasons. First, although genetic data have particular features, also other non-genetic data can be extremely sensitive. Second, having different regulatory requirements depending on the nature of data hinders the creation of comprehensible consent forms. Third, empirical evidence about public preferences concerning data reuse suggests that exceptional protection for genetic data alone is not justified. In this sense, it is claimed that regulation concerning data reuse should treat genetic data as important, but not exceptional.
Keywords: data protection; genetic data; genetic exceptionalism; research policy; secondary use.
Copyright © 2019 Martani, Geneviève, Pauli-Magnus, McLennan and Elger.
Figures
References
-
- Barton C., Kallem C., Van Dyke P., Mon D., Richesson R. (2011). “Demonstrating "Collect once, Use Many"–Assimilating Public Health Secondary Data Use Requirements into an Existing Domain Analysis Model,” in AMIA Annual Symposium Proceedings, vol. 2011 (American Medical Informatics Association; ), 98. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

