Achieving postprandial glucose control with lixisenatide improves glycemic control in patients with type 2 diabetes on basal insulin: a post-hoc analysis of pooled data
- PMID: 31956422
- PMCID: PMC6961286
- DOI: 10.1186/s40842-019-0088-5
Achieving postprandial glucose control with lixisenatide improves glycemic control in patients with type 2 diabetes on basal insulin: a post-hoc analysis of pooled data
Abstract
Background: To examine the impact on glycemic control of achieving postprandial glucose (PPG) target with lixisenatide, a once-daily glucagon-like peptide-1 receptor agonist approved in the US, in patients with uncontrolled type 2 diabetes (T2D) on basal insulin, an agent that primarily targets fasting plasma glucose.
Methods: A post hoc pooled analysis was conducted using clinical trial data extracted from the intent-to-treat subpopulation of patients with T2D who participated in the 24-week, phase 3, randomized, double-blind, placebo-controlled, 2-arm parallel-group, multicenter GetGoal-L (NCT00715624), GetGoal-Duo 1 (NCT00975286) and GetGoal-L Asia trials (NCT00866658).
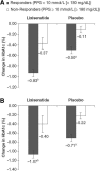
Results: Data from 587 lixisenatide-treated patients and 484 placebo-treated patients were included. Patients on lixisenatide were more likely to achieve a PPG target of < 10 mmol/L (< 180 mg/dL) than placebo-treated patients (P < 0.001), regardless of baseline fasting plasma glucose (FPG) levels. More importantly, those who reached the PPG target experienced a significantly greater reduction in mean HbA1c, were more likely to achieve HbA1c target of < 53 mmol/mol (< 7.0%), and experienced weight loss. Those outcomes were achieved with no significant differences in the risk of symptomatic hypoglycemia compared with placebo.
Conclusion: Compared with placebo, addition of lixisenatide to basal insulin improved HbA1c and reduced PPG, without increasing hypoglycemia risk. These findings highlight the importance of PPG control in the management of T2D, and provide evidence that adding an agent to basal insulin therapy that also impacts PPG has therapeutic value for patients who are not meeting glycemic targets.
Trial registration: NCT00715624. Registered 15 July 2008, NCT00975286. Registered 11 September 2009, NCT00866658. Registered 20 March 2009.
Keywords: Glucagon-like peptide-1 receptor; Glycemic targets; Lixisenatide; Post-prandial glucose; Type 2 diabetes.
© The Author(s). 2020.
Conflict of interest statement
Competing interestsJAD has been a consultant or served on the advisory board for Amgen Inc., Aspire Bariatrics, AstraZeneca, Boston Therapeutics, Eli Lilly & Co., GSK, Janssen Pharmaceuticals, Merck & Co., Inc., Novo Nordisk, and Valeritas; has been a speaker for AstraZeneca, Janssen, Merck-Serono, Novo Nordisk, and Takeda; and has received a research grant from AstraZeneca. WS, SP, and RB are employees and stockholders of Sanofi. LAL. has been a consultant for AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Janssen, Merck & Co., Inc., Novo Nordisk, Sanofi, and Servier; has received research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., GlaxoSmithKline, Janssen, Merck & Co., Inc., Novo Nordisk, and Sanofi; and has provided continuing medical education on behalf of AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Janssen, Merck & Co., Inc., Novo Nordisk, Sanofi, and Servier.
Figures


References
-
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53. - PubMed
-
- American Diabetes Association Standards of medical care in diabetes. Diabetes Care. 2019;42(Suppl 1):S1–S193. - PubMed
-
- International Diabetes Federation . IDF guideline on self-monitoring of blood glucose in non-insulin treated type 2 diabetes. 2011. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

