Wood-Derived Carbon Fibers Embedded with SnO x Nanoparticles as Anode Material for Lithium-Ion Batteries
- PMID: 31956425
- PMCID: PMC6957017
- DOI: 10.1002/gch2.201900048
Wood-Derived Carbon Fibers Embedded with SnO x Nanoparticles as Anode Material for Lithium-Ion Batteries
Abstract
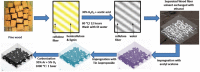
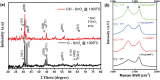
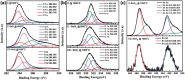
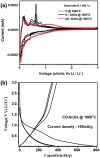
Carbon-SnO x composites are obtained by impregnating acetylacetone-treated, delignified wood fibers with tin precursor and successively carbonizing at 1000 °C in 95% argon and 5% oxygen. Scanning electron microscopy and nitrogen sorption studies (Brunauer-Emmett-Teller) show that acetylacetone treatment stabilizes the wood fiber structure during carbonization at 1000 °C and preserves the porous structural features. X-ray diffraction, transmission electron microscopy, and X-ray photoelectron spectroscopy studies show that the small amount of oxygen introduced in inert atmosphere passivates the surface of tin nanoparticles. The passivation process yields thermally and electrochemically stable SnO x particles embedded in carbon matrix. The resultant carbon-SnO x material with 16 wt% SnO x shows excellent electrochemical performance of rate capability from 0.1 to 10 A g-1 and cycling stability for 1000 cycles with Li-ion storage capacity of 280 mAh g-1 at a current density of 10 A g-1. The remarkable electrochemical performance of wood-derived carbon-SnO x composite is attributed to the reproduction of structural featured wood fibers to nanoscale in carbon-SnO x composite and controlled passivation of tin nanoparticles to yield SnO x nanoparticles.
Keywords: anode materials; carbon–tin composites; lithium‐ion batteries; tin oxide; wood fibers.
© 2019 The Authors. Published by WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Armand M., Tarascon J.‐M., Nature 2008, 451, 652. - PubMed
-
- Bruce P. G., Scrosati B., Tarascon J., Angew. Chem., Int. Ed. 2008, 16, 2930. - PubMed
-
- Lu L., Han X., Li J., Hua J., Ouyang M., J. Power Sources 2013, 226, 272.
-
- Zhu G. N., Wang Y. G., Xia Y. Y., Energy Environ. Sci. 2012, 5, 6652.
-
- Goriparti S., Miele E., De Angelis F., Di Fabrizio E., Zaccaria R. P., Capiglia C., J. Power Sources 2014, 257, 421.
LinkOut - more resources
Full Text Sources

