Efficient and New Production Methods of Chemicals and Liquid Fuels by Carbon Monoxide Hydrogenation
- PMID: 31956750
- PMCID: PMC6963990
- DOI: 10.1021/acsomega.9b03577
Efficient and New Production Methods of Chemicals and Liquid Fuels by Carbon Monoxide Hydrogenation
Abstract
Carbon monoxide (CO) hydrogenation is an important step for efficient utilization of carbon resources in C1 (one carbon) chemistry. Over recent years, this direction has been a hot research area in academia and industry and has also been one of the most challenging routes for the nonoil carbon resources utilization process. A large number of novel reaction routes and catalysts have been studied and reported. Efficient activation and directional conversion of CO are key aspects in the process of CO utilization. Furthermore, effectively activating C-O and C-C bond formation as well as controlling carbon chain growth is the current technical bottleneck of CO hydrogenation. This mini-review introduces the latest research progress for different catalyst systems and processes in CO hydrogenation and analyzes the factors that control the performance of catalysts in different reaction systems. Here, much focus is put on the synthesis of long-chain hydrocarbons, light olefins, C2+ oxygenates, and aromatics, essentially in comparison with the previous reports. Finally, the present challenges and future research directions have been discussed.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
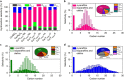
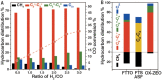

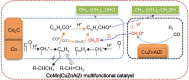

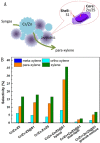
References
-
- Bao J.; Yang G.; Yoneyama Y.; Tsubaki N. Significant advances in C1 catalysis: highly efficient catalysts and catalytic reactions. ACS Catal. 2019, 9 (4), 3026–3053. 10.1021/acscatal.8b03924. - DOI
-
- Torres Galvis H. M.; De Jong K. P. Catalysts for production of lower olefins from synthesis gas: A review. ACS Catal. 2013, 3 (9), 2130–2149. 10.1021/cs4003436. - DOI
-
- Li J.; He Y.; Tan L.; Zhang P.; Peng X.; Oruganti A.; Yang G.; Abe H.; Wang Y.; Tsubaki N. Integrated tuneable synthesis of liquid fuels via Fischer–Tropsch technology. Nature Catalysis. 2018, 1, 787–793. 10.1038/s41929-018-0144-z. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
