Variants in NGLY1 lead to intellectual disability, myoclonus epilepsy, sensorimotor axonal polyneuropathy and mitochondrial dysfunction
- PMID: 31957011
- PMCID: PMC7078978
- DOI: 10.1111/cge.13706
Variants in NGLY1 lead to intellectual disability, myoclonus epilepsy, sensorimotor axonal polyneuropathy and mitochondrial dysfunction
Abstract
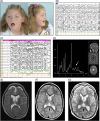
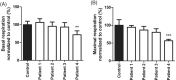
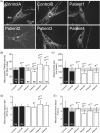
NGLY1 encodes the enzyme N-glycanase that is involved in the degradation of glycoproteins as part of the endoplasmatic reticulum-associated degradation pathway. Variants in this gene have been described to cause a multisystem disease characterized by neuromotor impairment, neuropathy, intellectual disability, and dysmorphic features. Here, we describe four patients with pathogenic variants in NGLY1. As the clinical features and laboratory results of the patients suggested a multisystem mitochondrial disease, a muscle biopsy had been performed. Biochemical analysis in muscle showed a strongly reduced ATP production rate in all patients, while individual OXPHOS enzyme activities varied from normal to reduced. No causative variants in any mitochondrial disease genes were found using mtDNA analysis and whole exome sequencing. In all four patients, variants in NGLY1 were identified, including two unreported variants (c.849T>G (p.(Cys283Trp)) and c.1067A>G (p.(Glu356Gly)). Western blot analysis of N-glycanase in muscle and fibroblasts showed a complete absence of N-glycanase. One patient showed a decreased basal and maximal oxygen consumption rates in fibroblasts. Mitochondrial morphofunction fibroblast analysis showed patient specific differences when compared to control cell lines. In conclusion, variants in NGLY1 affect mitochondrial energy metabolism which in turn might contribute to the clinical disease course.
Keywords: NGLY1; OXPHOS enzyme activity; Seahorse respirometry; Whole exome sequencing; mitochondrial disorders.
© 2020 The Authors. Clinical Genetics published by John Wiley & Sons Ltd.
Conflict of interest statement
J.S. is, next to this Radboudumc position, founding CEO of Khondrion. The company was not involved in this manuscript. W.J.H.K. is a scientific advisor of Khondrion BV (Nijmegen, the Netherlands) and of Fortify Therapeutics (Palo Alto, USA). These SMEs had no involvement in the data collection, analysis and interpretation, writing of the manuscript, and in the decision to submit the manuscript for publication.
Figures


References
-
- Rahman S. Mitochondrial disease and epilepsy. Dev Med Child Neurol. 2012;54:397‐406. - PubMed
-
- de Siqueira LF. Progressive myoclonic epilepsies: review of clinical, molecular and therapeutic aspects. J Neurol. 2010;257:1612‐1619. - PubMed
-
- Heeley J, Shinawi M. Multi‐systemic involvement in NGLY1‐related disorder caused by two novel mutations. Am J Med Genet A. 2015;167A:816‐820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

