Relaxation of Extracellular Matrix Forces Directs Crypt Formation and Architecture in Intestinal Organoids
- PMID: 31957249
- PMCID: PMC7274865
- DOI: 10.1002/adhm.201901214
Relaxation of Extracellular Matrix Forces Directs Crypt Formation and Architecture in Intestinal Organoids
Abstract
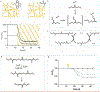
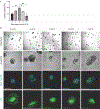
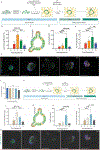
Intestinal organoid protocols rely on the use of extracellular scaffolds, typically Matrigel, and upon switching from growth to differentiation promoting media, a symmetry breaking event takes place. During this stage, the first bud like structures analogous to crypts protrude from the central body and differentiation ensues. While organoids provide unparalleled architectural and functional complexity, this sophistication is also responsible for the high variability and lack of reproducibility of uniform crypt-villus structures. If function follows form in organoids, such structural variability carries potential limitations for translational applications (e.g., drug screening). Consequently, there is interest in developing synthetic biomaterials to direct organoid growth and differentiation. It has been hypothesized that synthetic scaffold softening is necessary for crypt development, and these mechanical requirements raise the question, what compressive forces and subsequent relaxation are necessary for organoid maturation? To that end, allyl sulfide hydrogels are employed as a synthetic extracellular matrix mimic, but with photocleavable bonds that temporally regulate the material's bulk modulus. By varying the extent of matrix softening, it is demonstrated that crypt formation, size, and number per colony are functions of matrix softening. An understanding of the mechanical dependence of crypt architecture is necessary to instruct homogenous, reproducible organoids for clinical applications.
Keywords: adaptable materials; hydrogels; intestinal organoids; mechanosensing; photodegradation.
© 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

