Knockout of LASP1 in CXCR4 expressing CML cells promotes cell persistence, proliferation and TKI resistance
- PMID: 31957290
- PMCID: PMC7077607
- DOI: 10.1111/jcmm.14910
Knockout of LASP1 in CXCR4 expressing CML cells promotes cell persistence, proliferation and TKI resistance
Abstract
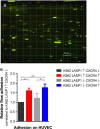
Chronic myeloid leukaemia (CML) is a clonal myeloproliferative stem cell disorder characterized by the constitutively active BCR-ABL tyrosine kinase. The LIM and SH3 domain protein 1 (LASP1) has recently been identified as a novel BCR-ABL substrate and is associated with proliferation, migration, tumorigenesis and chemoresistance in several cancers. Furthermore, LASP1 was shown to bind to the chemokine receptor 4 (CXCR4), thought to be involved in mechanisms of relapse. In order to identify potential LASP1-mediated pathways and related factors that may help to further eradicate minimal residual disease (MRD), the effect of LASP1 on processes involved in progression and maintenance of CML was investigated. The present data indicate that not only overexpression of CXCR4, but also knockout of LASP1 contributes to proliferation, reduced apoptosis and migration as well as increased adhesive potential of K562 CML cells. Furthermore, LASP1 depletion in K562 CML cells leads to decreased cytokine release and reduced NK cell-mediated cytotoxicity towards CML cells. Taken together, these results indicate that in CML, reduced levels of LASP1 alone and in combination with high CXCR4 expression may contribute to TKI resistance.
Keywords: BCR-ABL; CML; CXCR4; LASP1; nilotinib; precursor cells.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
EB and JF have received grants from the German Cancer Aid (Project numbers: 70112717 and 70112142). The authors declare that they have no conflicts of interests. Content has not been published in similar or the same form elsewhere.
Figures






 ) were administered unsuccessfully. As cytogenetics finally revealed the presence of Y253H, E255K and T315I mutations, ponatinib (dotted line) was initiated. Subsequently, the administered pre‐phase chemotherapy consisted of methotrexate, dexamethasone and cyclophosphamide (indicated as
) were administered unsuccessfully. As cytogenetics finally revealed the presence of Y253H, E255K and T315I mutations, ponatinib (dotted line) was initiated. Subsequently, the administered pre‐phase chemotherapy consisted of methotrexate, dexamethasone and cyclophosphamide (indicated as  ) according to German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia.44 Induction therapy had to be interrupted due to clinical deterioration. Finally, the patient underwent conditioning therapy with treosulfan, fludarabine and antithymocyte globulin45 followed by allogenic peripheral blood stem cell transplantation of 4.1 × 106 CD34+ cells/kg bodyweight (indicated as ▼) from an unrelated male, human leucocyte antigen allele matched 10/10 donor. After peripheral blood stem cell transplantation, BCR‐ABL levels declined, while LASP1 levels increased. Due to a renewed blast crisis (and concomitant lowered LASP1 levels), the patient died 83 d after SCT (indicated as
) according to German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia.44 Induction therapy had to be interrupted due to clinical deterioration. Finally, the patient underwent conditioning therapy with treosulfan, fludarabine and antithymocyte globulin45 followed by allogenic peripheral blood stem cell transplantation of 4.1 × 106 CD34+ cells/kg bodyweight (indicated as ▼) from an unrelated male, human leucocyte antigen allele matched 10/10 donor. After peripheral blood stem cell transplantation, BCR‐ABL levels declined, while LASP1 levels increased. Due to a renewed blast crisis (and concomitant lowered LASP1 levels), the patient died 83 d after SCT (indicated as  ) despite further doses of cyclophosphamide (indicated as
) despite further doses of cyclophosphamide (indicated as  ). The provenience and preparation of blood samples have been described before.9 CML, chronic myeloid leukaemia; CT, chemotherapy; LASP1, LIM and SH3 domain protein 1; PBSCT, peripheral blood stem cell transplantation; TKI, tyrosine kinase inhibitor
). The provenience and preparation of blood samples have been described before.9 CML, chronic myeloid leukaemia; CT, chemotherapy; LASP1, LIM and SH3 domain protein 1; PBSCT, peripheral blood stem cell transplantation; TKI, tyrosine kinase inhibitorReferences
-
- Tantravahi SK, Guthula RS, O'Hare T, Deininger MW. Minimal residual disease eradication in CML: does it really matter? Curr Hematol Malig Rep. 2017;12:495‐505. - PubMed
-
- Rea D, Cayuela JM. Treatment‐free remission in patients with chronic myeloid leukemia. Int J Hematol. 2018;108:355‐364. - PubMed
-
- Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029‐1035. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

