Altered corticolimbic connectivity reveals sex-specific adolescent outcomes in a rat model of early life adversity
- PMID: 31958061
- PMCID: PMC7010412
- DOI: 10.7554/eLife.52651
Altered corticolimbic connectivity reveals sex-specific adolescent outcomes in a rat model of early life adversity
Abstract
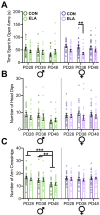
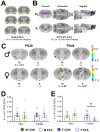
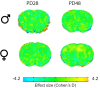
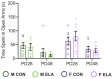
Exposure to early-life adversity (ELA) increases the risk for psychopathologies associated with amygdala-prefrontal cortex (PFC) circuits. While sex differences in vulnerability have been identified with a clear need for individualized intervention strategies, the neurobiological substrates of ELA-attributable differences remain unknown due to a paucity of translational investigations taking both development and sex into account. Male and female rats exposed to maternal separation ELA were analyzed with anterograde tracing from basolateral amygdala (BLA) to PFC to identify sex-specific innervation trajectories through juvenility (PD28) and adolescence (PD38;PD48). Resting-state functional connectivity (rsFC) was assessed longitudinally (PD28;PD48) in a separate cohort. All measures were related to anxiety-like behavior. ELA-exposed rats showed precocial maturation of BLA-PFC innervation, with females affected earlier than males. ELA also disrupted maturation of female rsFC, with enduring relationships between rsFC and anxiety-like behavior. This study is the first providing both anatomical and functional evidence for sex- and experience-dependent corticolimbic development.
Keywords: adolescence; basolateral amygdala; behavior; developmental biology; innervation; maternal separation; neuroscience; prefrontal cortex; rat.
Plain language summary
Having a traumatic childhood increases the risk a person will develop anxiety disorders later in life. Early life adversity affects men and women differently, but scientists do not yet know why. Learning more could help scientists develop better ways to prevent or treat anxiety disorders in men and women who experienced childhood trauma. Anxiety occurs when threat-detecting brain circuits turn on. These circuits begin working in infancy, and during childhood and adolescence, experiences shape the brain to hone the body’s responses to perceived threats. Two areas of the brain that are important hubs for anxiety-related brain circuits include the basolateral amygdala (BLA) and the prefrontal cortex (PFC). Now, Honeycutt et al. show that rats that experience early life adversity develop stronger connections between the BLA and PFC, and these changes occur earlier in female rats. In the experiments, one group of rats was repeatedly separated from their mothers and littermates (an early life trauma), while a second group was not. Honeycutt et al. examined the connections between the BLA and PFC in the two groups at three different time periods during their development: the juvenile stage, early adolescence, and late adolescence. The experiments showed stronger connections between the BLA and PFC begin to appear earlier in juvenile traumatized female rats. But these changes did not appear in their male counterparts until adolescence. Lastly, the rats that developed these strengthened BLA-PFC connections also behaved more anxiously later in life. This may mean that the ideal timing for interventions may be different for males and females. More work is needed to see if these results translate to humans and then to find the best times and methods to help people who experienced childhood trauma.
© 2020, Honeycutt et al.
Conflict of interest statement
JH, CD, SP, MS, XC, PK, MC, HB No competing interests declared, CF has a financial interest in Animal Imaging Research, the company that makes the rat imaging system
Figures








References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

