Pine Bark Polyphenolic Extract Attenuates Amyloid-β and Tau Misfolding in a Model System of Alzheimer's Disease Neuropathology
- PMID: 31958081
- PMCID: PMC8162892
- DOI: 10.3233/JAD-190543
Pine Bark Polyphenolic Extract Attenuates Amyloid-β and Tau Misfolding in a Model System of Alzheimer's Disease Neuropathology
Erratum in
-
Pine Bark Polyphenolic Extract Attenuates Amyloid-β and Tau Misfolding in a Model System of Alzheimer's Disease Neuropathology.J Alzheimers Dis. 2020;77(1):457. doi: 10.3233/JAD-209007. J Alzheimers Dis. 2020. PMID: 32894246 No abstract available.
Abstract
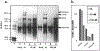
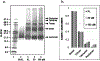
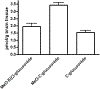
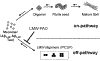
Plant-derived polyphenolic compounds possess diverse biological activities, including strong anti-oxidant, anti-inflammatory, anti-microbial, and anti-tumorigenic activities. There is a growing interest in the development of polyphenolic compounds for preventing and treating chronic and degenerative diseases, such as cardiovascular disorders, cancer, and neurological diseases including Alzheimer's disease (AD). Two neuropathological changes of AD are the appearance of neurofibrillary tangles containing tau and extracellular amyloid deposits containing amyloid-β protein (Aβ). Our laboratory and others have found that polyphenolic preparations rich in proanthocyanidins, such as grape seed extract, are capable of attenuating cognitive deterioration and reducing brain neuropathology in animal models of AD. Oligopin is a pine bark extract composed of low molecular weight proanthocyanidins oligomers (LMW-PAOs), including flavan-3-ol units such as catechin (C) and epicatechin (EC). Based on the ability of its various components to confer resilience to the onset of AD, we tested whether oligopin can specifically prevent or attenuate the progression of AD dementia preclinically. We also explored the underlying mechanism(s) through which oligopin may exert its biological activities. Oligopin inhibited oligomer formation of not only Aβ1-40 and Aβ1-42, but also tau in vitro. Our pharmacokinetics analysis of metabolite accumulation in vivo resulted in the identification of Me-EC-O-β-Glucuronide, Me-(±)-C-O-β-glucuronide, EC-O-β-glucuronide, and (±)-C-O-β-glucuronide in the plasma of mice. These metabolites are primarily methylated and glucuronidated C and EC conjugates. The studies conducted provide the necessary impetus to design future clinical trials with bioactive oligopin to prevent both prodromal and residual forms of AD.
Keywords: Alzheimer’s disease; amyloid β-peptide; polyphenols; tau.
Figures


Similar articles
-
Cognitive-Enhancing Effects of a Polyphenols-Rich Extract from Fruits without Changes in Neuropathology in an Animal Model of Alzheimer's Disease.J Alzheimers Dis. 2017;55(1):115-135. doi: 10.3233/JAD-160281. J Alzheimers Dis. 2017. PMID: 27662290
-
Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer's disease.J Neurosci. 2008 Jun 18;28(25):6388-92. doi: 10.1523/JNEUROSCI.0364-08.2008. J Neurosci. 2008. PMID: 18562609 Free PMC article.
-
The Amazon rain forest plant Uncaria tomentosa (cat's claw) and its specific proanthocyanidin constituents are potent inhibitors and reducers of both brain plaques and tangles.Sci Rep. 2019 Feb 6;9(1):561. doi: 10.1038/s41598-019-38645-0. Sci Rep. 2019. PMID: 30728442 Free PMC article.
-
Novel role of red wine-derived polyphenols in the prevention of Alzheimer's disease dementia and brain pathology: experimental approaches and clinical implications.Planta Med. 2012 Oct;78(15):1614-9. doi: 10.1055/s-0032-1315377. Epub 2012 Sep 21. Planta Med. 2012. PMID: 23023952 Review.
-
Review on Alzheimer's disease: Inhibition of amyloid beta and tau tangle formation.Int J Biol Macromol. 2021 Jan 15;167:382-394. doi: 10.1016/j.ijbiomac.2020.11.192. Epub 2020 Dec 2. Int J Biol Macromol. 2021. PMID: 33278431 Review.
Cited by
-
Can Natural Products Exert Neuroprotection without Crossing the Blood-Brain Barrier?Int J Mol Sci. 2021 Mar 25;22(7):3356. doi: 10.3390/ijms22073356. Int J Mol Sci. 2021. PMID: 33805947 Free PMC article. Review.
-
Investigating the Impact of Sorghum on Tau Protein Phosphorylation and Mitochondrial Dysfunction Modulation in Alzheimer's Disease: An In Vitro Study.Nutrients. 2025 Jan 30;17(3):516. doi: 10.3390/nu17030516. Nutrients. 2025. PMID: 39940374 Free PMC article.
-
Purple sweet potato delphinidin-3-rutin represses glioma proliferation by inducing miR-20b-5p/Atg7-dependent cytostatic autophagy.Mol Ther Oncolytics. 2022 Jul 31;26:314-329. doi: 10.1016/j.omto.2022.07.007. eCollection 2022 Sep 15. Mol Ther Oncolytics. 2022. PMID: 36090477 Free PMC article.
-
Protective Effects of Ginkgolide on a Cellular Model of Alzheimer's Disease via Suppression of the NF-κB Signaling Pathway.Appl Biochem Biotechnol. 2022 Jun;194(6):2448-2464. doi: 10.1007/s12010-022-03828-5. Epub 2022 Feb 7. Appl Biochem Biotechnol. 2022. PMID: 35129804 Free PMC article.
References
-
- Cummings JL (2004) Alzheimer’s disease. N Engl J Med 351, 56–67. - PubMed
-
- Selkoe DJ (2001) Alzheimer’s disease: Genes, proteins, and therapy. Physiol Rev 81, 741–766. - PubMed
-
- Hardy J, Allsop D (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 12, 383–388. - PubMed
-
- Haass C, Schlossmacher MG, Hung AY, Vigopelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, Selkoe DJ (1992) Amyloid β-peptide is produced by cultured-cells during normal metabolism. Nature 359, 322–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

