Comparing Adaptive Radiations Across Space, Time, and Taxa
- PMID: 31958131
- PMCID: PMC7931853
- DOI: 10.1093/jhered/esz064
Comparing Adaptive Radiations Across Space, Time, and Taxa
Abstract
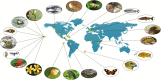
Adaptive radiation plays a fundamental role in our understanding of the evolutionary process. However, the concept has provoked strong and differing opinions concerning its definition and nature among researchers studying a wide diversity of systems. Here, we take a broad view of what constitutes an adaptive radiation, and seek to find commonalities among disparate examples, ranging from plants to invertebrate and vertebrate animals, and remote islands to lakes and continents, to better understand processes shared across adaptive radiations. We surveyed many groups to evaluate factors considered important in a large variety of species radiations. In each of these studies, ecological opportunity of some form is identified as a prerequisite for adaptive radiation. However, evolvability, which can be enhanced by hybridization between distantly related species, may play a role in seeding entire radiations. Within radiations, the processes that lead to speciation depend largely on (1) whether the primary drivers of ecological shifts are (a) external to the membership of the radiation itself (mostly divergent or disruptive ecological selection) or (b) due to competition within the radiation membership (interactions among members) subsequent to reproductive isolation in similar environments, and (2) the extent and timing of admixture. These differences translate into different patterns of species accumulation and subsequent patterns of diversity across an adaptive radiation. Adaptive radiations occur in an extraordinary diversity of different ways, and continue to provide rich data for a better understanding of the diversification of life.
© The American Genetic Association 2020. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Ackerly DD, Schwilk DW, Webb CO. 2006. Niche evolution and adaptive radiation: testing the order of trait divergence. Ecology. 87:S50–S61. - PubMed
-
- Agrawal AF, Feder JL, Nosil P. 2011. Ecological divergence and the origins of intrinsic postmating isolation with gene flow. Int J Ecol. 2011.
-
- Arbogast BS, Drovetski SV, Curry RL, Boag PT, Seutin G, Grant PR, Grant BR, Anderson DJ. 2006. The origin and diversification of Galapagos mockingbirds. Evolution. 60:370–382. - PubMed

