First-line and second-line treatment of patients with metastatic pancreatic adenocarcinoma in routine clinical practice across Europe: a retrospective, observational chart review study
- PMID: 31958291
- PMCID: PMC7003396
- DOI: 10.1136/esmoopen-2019-000587
First-line and second-line treatment of patients with metastatic pancreatic adenocarcinoma in routine clinical practice across Europe: a retrospective, observational chart review study
Abstract
Background: Treatment of metastatic pancreatic adenocarcinoma (mPAC) relies on chemotherapeutic regimens. We investigated patterns of first-line and second-line treatment choices, their geographical variation between European countries, and alignment with current European recommendations.
Methods: This retrospective, observational chart review study was conducted between July 2014 and January 2016. Physicians were recruited from nine European countries. Patient data were collected in electronic patient record forms (PRFs) by physicians managing patients with mPAC. Patients with a current mPAC diagnosis aged ≥18 years old who had completed first-line therapy during the study period were included.
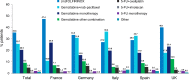
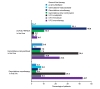
Results: Participating physicians (n=225) completed 2565 PRFs. The vast majority of PRFs were from France, Germany, Italy, Spain and the UK. Most patients (86.6%) had stage IV disease at diagnosis. The most common first-line treatments were FOLFIRINOX (5-fluorouracil, leucovorin/folinic acid, irinotecan and oxaliplatin) (35.6%), gemcitabine+nab-paclitaxel (25.7%) and gemcitabine monotherapy (20.5%). Physicians in France and the UK prescribed FOLFIRINOX more frequently than gemcitabine+nab-paclitaxel. Gemcitabine-based therapies were more widely used at second-line, although 5-fluorouracil-based therapies were preferred in Italy and Spain, where gemcitabine-based treatments were more frequently selected for first-line. For patients receiving first-line modified FOLFIRINOX, second-line gemcitabine monotherapy was preferred in the overall population (45.9%).
Conclusion: Although treatment choices for patients with mPAC varied between countries, they align with current European guidelines. Factors including drug availability, reimbursement, patient characteristics, physician preference and prior first-line therapy affect treatment choices. Approved, recommended therapies for patients who progress following first-line treatment are lacking. These findings may influence the development of effective treatment plans, potentially improving future patient outcomes.
Keywords: 5-fluorouracil; Western Europe; albumin-bound paclitaxel; gemcitabine; pancreatic cancer.
© Author (s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. Published by BMJ on behalf of the European Society for Medical Oncology.
Conflict of interest statement
Competing interests: JT has acted as a consultant or speaker for Amgen, Roche, Merck Serono, Celgene, Shire, MSD, Lilly, Pierre Fabre, Sanofi, Sirtex and Servier. GP has acted as a consultant or speaker for Shire, Celgene, Merck Serono, Roche, Amgen, Sanofi, Lilly, Bayer, Servier, Bristol-Myers Squibb, MSD, Taiho and Halozyme. DM has received research funding from Shire, Incyte, Evotec and Celgene; and acted as a consultant for Lilly, Shire, Evotec, Servier, Baxter and Incyte. AC has acted as a consultant or advisor for Roche, Merck, MSD, Servier, Shire, Celgene and Bayer; and has received travel expenses from Merck, Celgene and Bristol-Myers Squibb. CBW has received research funding from Roche; and has acted as a consultant or advisor for Roche, Shire/Baxalta, Bayer, Ipsen, Rafael Pharmaceuticals, Celgene and Redhill. ND and AF are employees of Genactis, a company that received funding from Shire to support data collection for this study. TM has acted as an advisor or speaker for Baxalta, Celgene, Genzyme, H3 Biomedicine, QED, Roche, Sanofi and Shire; and has received support for travel and accommodation from Bayer, H3 Biomedicine, Merck and Sanofi. She has received research funding from Agios, Aslan, AstraZeneca, Bayer, Celgene, Genentech, Halozyme, Immunomedics, Lilly, Merrimack, Millennium, Novartis, Novocure, Pfizer, Pharmacyclics and Roche.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

