Concise whole blood transcriptional signatures for incipient tuberculosis: a systematic review and patient-level pooled meta-analysis
- PMID: 31958400
- PMCID: PMC7113839
- DOI: 10.1016/S2213-2600(19)30282-6
Concise whole blood transcriptional signatures for incipient tuberculosis: a systematic review and patient-level pooled meta-analysis
Abstract
Background: Multiple blood transcriptional signatures have been proposed for identification of active and incipient tuberculosis. We aimed to compare the performance of systematically identified candidate signatures for incipient tuberculosis and to benchmark these against WHO targets.
Methods: We did a systematic review and individual participant data meta-analysis. We searched Medline and Embase for candidate whole blood mRNA signatures discovered with the primary objective of diagnosis of active or incipient tuberculosis, compared with controls who were healthy or had latent tuberculosis infection. We tested the performance of eligible signatures in whole blood transcriptomic datasets, in which sampling before tuberculosis diagnosis was done and time to disease was available. Culture-confirmed and clinically or radiologically diagnosed pulmonary or extrapulmonary tuberculosis cases were included. Non-progressor (individuals who remained tuberculosis-free during follow-up) samples with less than 6 months of follow-up from the date of sample collection were excluded, as were participants with prevalent tuberculosis and those who received preventive therapy. Scores were calculated for candidate signatures for each participant in the pooled dataset. Receiver operating characteristic curves, sensitivities, and specificities were examined using prespecified intervals to tuberculosis (<3 months, <6 months, <1 year, and <2 years) from sample collection. This study is registered with PROSPERO, number CRD42019135618.
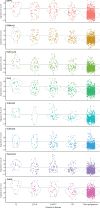
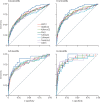
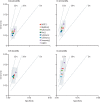
Results: We tested 17 candidate mRNA signatures in a pooled dataset from four eligible studies comprising 1126 samples. This dataset included 183 samples from 127 incipient tuberculosis cases in South Africa, Ethiopia, The Gambia, and the UK. Eight signatures (comprising 1-25 transcripts) that predominantly reflect interferon and tumour necrosis factor-inducible gene expression, had equivalent diagnostic accuracy for incipient tuberculosis over a 2-year period with areas under the receiver operating characteristic curves ranging from 0·70 (95% CI 0·64-0·76) to 0·77 (0·71-0·82). The sensitivity of all eight signatures declined with increasing disease-free time interval. Using a threshold derived from two SDs above the mean of uninfected controls to prioritise specificity and positive-predictive value, the eight signatures achieved sensitivities of 24·7-39·9% over 24 months and of 47·1-81·0% over 3 months, with corresponding specificities of more than 90%. Based on pre-test probability of 2%, the eight signatures achieved positive-predictive values ranging from 6·8-9·4% over 24 months and 11·2-14·4% over 3 months. When using biomarker thresholds maximising sensitivity and specificity with equal weighting to both, no signature met the minimum WHO target product profile parameters for incipient tuberculosis biomarkers over a 2-year period.
Interpretation: Blood transcriptional biomarkers reflect short-term risk of tuberculosis and only exceed WHO benchmarks if applied to 3-6-month intervals. Serial testing among carefully selected target groups might be required for optimal implementation of these biomarkers.
Funding: Wellcome Trust and National Institute for Health Research.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Headway made towards biosignatures for incipient tuberculosis.Lancet Respir Med. 2020 Apr;8(4):328-330. doi: 10.1016/S2213-2600(19)30355-8. Epub 2020 Jan 17. Lancet Respir Med. 2020. PMID: 31958399 Free PMC article. No abstract available.
References
-
- WHO . World Health Organization; 2018. Global Tuberculosis Report 2018.https://www.who.int/tb/publications/global_report/en/
-
- WHO . World Health Organization; 2015. The End TB Strategy.http://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

