Genetics of Treatment Outcomes in Major Depressive Disorder: Present and Future
- PMID: 31958900
- PMCID: PMC7006978
- DOI: 10.9758/cpn.2020.18.1.1
Genetics of Treatment Outcomes in Major Depressive Disorder: Present and Future
Abstract
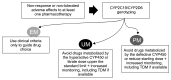
Pharmacogenetic testing is a useful and increasingly widespread tool to assist in antidepressant prescription. More than ten antidepressants (including tricyclics, selective serotonin reuptake inhibitors and venlafaxine) have already genetic biomarkers of response/side effects in clinical guidelines and drug labels. These are represented by functional genetic variants in genes coding for cytochrome enzymes (CYP2D6 and CYP2C19). Depending on the predicted metabolic activity, guidelines provide recommendations on drug choice and dosing. Despite not conclusive, the current evidence suggests that testing can be useful in patients who did not respond or tolerate at least one previous pharmacotherapy. However, the current recommendations are based on pharmacokinetic genes only (CYP450 enzymes), while pharmacodynamic genes (modulating antidepressant mechanisms of action in the brain) are still being studied because of their greater complexity. This may be captured by polygenic risk scores, which reflect the cumulative contribution of many genetic variants to a trait, and they may provide future clinical applications of pharmacogenetics. A more extensive use of genotyping in clinical practice may lead to improvement in treatment outcomes thanks to personalized treatments, but possible ethical issues and disparities should be taken into account and prevented.
Keywords: Antidepressant; Genetics; Genome-wide association study; Major depressive disorder; Polygenic risk score; Precision psychiatry..
Conflict of interest statement
Prof. Alessandro Serretti is or has been consultant/speaker for: Abbott, Abbvie, Angelini, Astra Zeneca, Clinical Data, Boheringer, Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Innovapharma, Italfarmaco, Janssen, Lundbeck, Naurex, Pfizer, Polifarma, Sanofi, Servier.
Chiara Fabbri has no potential conflicts of interest.
Figures


References
-
- National Human Genome Research Institute. DNA sequencing costs: data [Internet] Bethesda: National Institute of Health; 2017. [cited at 2017 Nov 27]. Available from: https://www.genome.gov/sequencingcostsdata/
LinkOut - more resources
Full Text Sources

