Changes in Cardiovascular Biomarkers With Breast Cancer Therapy and Associations With Cardiac Dysfunction
- PMID: 31959034
- PMCID: PMC7033834
- DOI: 10.1161/JAHA.119.014708
Changes in Cardiovascular Biomarkers With Breast Cancer Therapy and Associations With Cardiac Dysfunction
Abstract
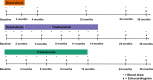
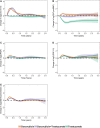
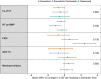
Background We examined the longitudinal associations between changes in cardiovascular biomarkers and cancer therapy-related cardiac dysfunction (CTRCD) in patients with breast cancer treated with cardotoxic cancer therapy. Methods and Results Repeated measures of high-sensitivity cardiac troponin T (hs-cTnT), NT-proBNP (N-terminal pro-B-type natriuretic peptide), myeloperoxidase, placental growth factor, and growth differentiation factor 15 were assessed longitudinally in a prospective cohort of 323 patients treated with anthracyclines and/or trastuzumab followed over a maximum of 3.7 years with serial echocardiograms. CTRCD was defined as a ≥10% decline in left ventricular ejection fraction to a value <50%. Associations between changes in biomarkers and left ventricular ejection fraction were evaluated in repeated-measures linear regression models. Cox regression models assessed the associations between biomarkers and CTRCD. Early increases in all biomarkers occurred with anthracycline-based regimens. hs-cTnT levels >14 ng/L at anthracycline completion were associated with a 2-fold increased CTRCD risk (hazard ratio, 2.01; 95% CI, 1.00-4.06). There was a modest association between changes in NT-proBNP and left ventricular ejection fraction in the overall cohort; this was most pronounced with sequential anthracycline and trastuzumab (1.1% left ventricular ejection fraction decline [95% CI, -1.8 to -0.4] with each NT-proBNP doubling). Increases in NT-proBNP were also associated with CTRCD (hazard ratio per doubling, 1.56; 95% CI, 1.32-1.84). Increases in myeloperoxidase were associated with CTRCD in patients who received sequential anthracycline and trastuzumab (hazard ratio per doubling, 1.28; 95% CI, 1.04-1.58). Conclusions Cardiovascular biomarkers may play an important role in CTRCD risk prediction in patients with breast cancer who receive cardiotoxic cancer therapy, particularly in those treated with sequential anthracycline and trastuzumab therapy. Clinical Trial Registration URL: https://www.clinicaltrials.gov/. Unique identifier: NCT01173341.
Keywords: biomarker; cardiomyopathy; cardiotoxicity; cardio‐oncology.
Figures
References
-
- Abdel‐Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, Fung K, Anderson GM. A population‐based study of cardiovascular mortality following early‐stage breast cancer. JAMA Cardiol. 2017;2:88–93. - PubMed
-
- Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM. Prevention of high‐dose chemotherapy‐induced cardiotoxicity in high‐risk patients by angiotensin‐converting enzyme inhibition. Circulation. 2006;114:2474–2481. - PubMed
-
- Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz‐Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff‐Brenkenhoff F, Bratland A, Storas TH, Hagve TA, Rosjo H, Steine K, Geisler J, Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo‐controlled, double‐blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680. - PMC - PubMed
-
- Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: american society of clinical oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. - PubMed
-
- Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM; ESC Scientific Document Group . 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

