Immunogold labeling of synaptic vesicle proteins in developing hippocampal neurons
- PMID: 31959215
- PMCID: PMC6971973
- DOI: 10.1186/s13041-020-0549-x
Immunogold labeling of synaptic vesicle proteins in developing hippocampal neurons
Abstract
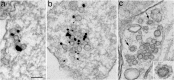
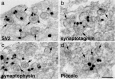
Synaptic vesicles (SV) contain high concentrations of specific proteins. How these proteins are transported from soma to synapses, and how they become concentrated at SV clusters at presynaptic terminals were examined by immunogold electron microscopy in dissociated rat hippocampal neurons at 3-6 days in culture, a developmental stage when axonal transport of SV proteins is robust. In neuronal somas, labels for the SV integral membrane proteins (synaptophysin, SV2, VAMP/synaptobrevin, and synaptotagmin) were localized at Golgi complexes and other membranous structures that were dispersed in the cytoplasm as individual vesicle/vacuoles. These vesicles/vacuoles became aggregated in axons, with the size of aggregates ranging from 0.2 to 2 μm in length. Pleomorphic vesicle/vacuoles within the aggregate were typically larger (50-300 nm) than SVs, which were uniform in size at ~ 40 nm. These pleomorphic vesicles/vacuoles are probably transport cargos carrying SV integral membrane proteins from the soma, and then are preferentially sorted into axons at early developmental stages. Serial thin sections of young axons indicated that many labeled aggregates were not synaptic, and in fact, some of these axons were without dendritic contacts. In contrast, labels for two SV-associated proteins, synapsin I and α-synuclein, were not localized at the Golgi complexes or associated with membranous structures in the soma, but were dispersed in the cytoplasm. However, these SV-associated proteins became highly concentrated on clusters of SV-like vesicles in axons, and such clusters were already distinctive in axons as early as 3 days in culture. These clusters consisted of ~ 4-30 vesicles in single thin sections, and the vesicles were of a uniform size (~ 40 nm). Serial sectioning analysis showed that these clusters could be part of nascent synapses or exist in axons without any dendritic contact. Importantly, the vesicles were intensely labeled for SV integral membrane proteins as well as SV-associated proteins. Thus, these EM observations reveal that the two groups of proteins, SV integral membrane and SV-associated, proceed through different routes of biosynthesis and axon transport, and are only sorted into the same final compartment, SV clusters, when they are in the axons.
Keywords: Active zone cytomatrix; Axon transport; Electron microscopy; SV2; Synapsin; Synaptophysin.
Conflict of interest statement
The author declares that she has no competing interests.
Figures







Similar articles
-
Synaptic vesicle proteins and early endosomes in cultured hippocampal neurons: differential effects of Brefeldin A in axon and dendrites.J Cell Biol. 1993 Sep;122(6):1207-21. doi: 10.1083/jcb.122.6.1207. J Cell Biol. 1993. PMID: 8376458 Free PMC article.
-
Ultrastructural localization of active zone and synaptic vesicle proteins in a preassembled multi-vesicle transport aggregate.Neuroscience. 2007 Dec 12;150(3):575-84. doi: 10.1016/j.neuroscience.2007.09.031. Epub 2007 Sep 19. Neuroscience. 2007. PMID: 17977664 Free PMC article.
-
Snap-25 is polarized to axons and abundant along the axolemma: an immunogold study of intact neurons.J Neurocytol. 2000 Jan;29(1):67-77. doi: 10.1023/a:1007168231323. J Neurocytol. 2000. PMID: 11068335
-
Traffic of synaptic vesicle proteins in polarized and nonpolarized cells.J Cell Sci Suppl. 1993;17:93-100. doi: 10.1242/jcs.1993.supplement_17.14. J Cell Sci Suppl. 1993. PMID: 8144709 Review.
-
The role of lipid binding for the targeting of synaptic proteins into synaptic vesicles.BMB Rep. 2009 Jan 31;42(1):1-5. doi: 10.5483/bmbrep.2009.42.1.001. BMB Rep. 2009. PMID: 19192386 Review.
Cited by
-
Ultrastructural characterization of hippocampal inhibitory synapses under resting and stimulated conditions.Mol Brain. 2024 Oct 22;17(1):76. doi: 10.1186/s13041-024-01151-0. Mol Brain. 2024. PMID: 39438991 Free PMC article.
-
Impaired Aggrephagy, Interrupted Vesicular Trafficking, and Cellular Stress, Lead to Protein Aggregation, and Synaptic Dysfunction in Cerebellum of Children and Adults with Idiopathic Autism.Cerebellum. 2025 Aug 8;24(5):140. doi: 10.1007/s12311-025-01880-5. Cerebellum. 2025. PMID: 40779003 Free PMC article.
-
Presynaptic perspective: Axonal transport defects in neurodevelopmental disorders.J Cell Biol. 2024 Jun 3;223(6):e202401145. doi: 10.1083/jcb.202401145. Epub 2024 Apr 3. J Cell Biol. 2024. PMID: 38568173 Free PMC article. Review.
-
Whole-Transcriptome Analysis of Repeated Low-Level Sarin-Exposed Rat Hippocampus and Identification of Cerna Networks to Investigate the Mechanism of Sarin-Induced Cognitive Impairment.Biology (Basel). 2023 Apr 20;12(4):627. doi: 10.3390/biology12040627. Biology (Basel). 2023. PMID: 37106826 Free PMC article.
-
LRK-1/LRRK2 and AP-3 regulate trafficking of synaptic vesicle precursors through active zone protein SYD-2/Liprin-α.PLoS Genet. 2024 May 9;20(5):e1011253. doi: 10.1371/journal.pgen.1011253. eCollection 2024 May. PLoS Genet. 2024. PMID: 38722918 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

