LRIT3 is Required for Nyctalopin Expression and Normal ON and OFF Pathway Signaling in the Retina
- PMID: 31959619
- PMCID: PMC7031853
- DOI: 10.1523/ENEURO.0002-20.2020
LRIT3 is Required for Nyctalopin Expression and Normal ON and OFF Pathway Signaling in the Retina
Abstract
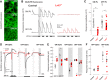
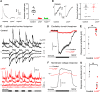
The first retinal synapse, photoreceptor→bipolar cell (BC), is both anatomically and functionally complex. Within the same synaptic region, a change in presynaptic glutamate release is sensed by both ON BCs (DBCs) via the metabotropic glutamate receptor 6 (mGluR6), and OFF BCs (HBCs) via ionotropic glutamate receptors to establish parallel signaling pathways that preferentially encode light increments (ON) or decrements (OFF), respectively. The synaptic structural organization of ON and OFF-type BCs at the photoreceptor terminal differs. DBCs make an invaginating synapse that contains a diverse but incompletely understood complex of interacting proteins (signalplex). HBCs make primarily flat contacts that contain an apparent different set of proteins that is equally uncharacterized. LRIT3 is a synaptic protein known to be essential for ON pathway visual function. In both male and female mice, we demonstrate that LRIT3 interacts with and is required for expression of nyctalopin, and thus TRPM1 at all DBC dendritic tips, but DBC signalplex components are not required for LRIT3 expression. Using whole-cell and multielectrode array (MEA) electrophysiology and glutamate imaging, we demonstrate that the loss of LRIT3 impacts both ON and OFF signaling pathway function. Without LRIT3, excitatory input to type 1 BCs is reduced, as are the visually evoked responses of many OFF retinal ganglion cells (RGCs). We conclude that the absence of LRIT3 expression disrupts excitatory input to OFF BCs and, thus disrupts the normal function of OFF RGCs.
Keywords: CSNB; LRIT3; glutamate dynamics; night blindness; nyctalopin.
Copyright © 2020 Hasan et al.
Figures



Similar articles
-
LRIT3 Differentially Affects Connectivity and Synaptic Transmission of Cones to ON- and OFF-Bipolar Cells.Invest Ophthalmol Vis Sci. 2017 Mar 1;58(3):1768-1778. doi: 10.1167/iovs.16-20745. Invest Ophthalmol Vis Sci. 2017. PMID: 28334377 Free PMC article.
-
Presynaptic Expression of LRIT3 Transsynaptically Organizes the Postsynaptic Glutamate Signaling Complex Containing TRPM1.Cell Rep. 2019 Jun 11;27(11):3107-3116.e3. doi: 10.1016/j.celrep.2019.05.056. Cell Rep. 2019. PMID: 31189098 Free PMC article.
-
LRIT3 expression in cone photoreceptors restores post-synaptic bipolar cell signalplex assembly and partial function in Lrit3 -/- mice.iScience. 2023 Mar 24;26(4):106499. doi: 10.1016/j.isci.2023.106499. eCollection 2023 Apr 21. iScience. 2023. PMID: 37091241 Free PMC article.
-
Properties and functions of TRPM1 channels in the dendritic tips of retinal ON-bipolar cells.Eur J Cell Biol. 2015 Jul-Sep;94(7-9):420-7. doi: 10.1016/j.ejcb.2015.06.005. Epub 2015 Jun 3. Eur J Cell Biol. 2015. PMID: 26111660 Review.
-
Comparisons of structural and functional abnormalities in mouse b-wave mutants.J Physiol. 2008 Sep 15;586(18):4385-92. doi: 10.1113/jphysiol.2008.159327. Epub 2008 Jul 24. J Physiol. 2008. PMID: 18653656 Free PMC article. Review.
Cited by
-
A retinal origin of nystagmus-a perspective.Front Ophthalmol (Lausanne). 2023 Jun 8;3:1186280. doi: 10.3389/fopht.2023.1186280. eCollection 2023. Front Ophthalmol (Lausanne). 2023. PMID: 38983059 Free PMC article.
-
Small Leucine-Rich Proteoglycans (SLRPs) in the Retina.Int J Mol Sci. 2021 Jul 7;22(14):7293. doi: 10.3390/ijms22147293. Int J Mol Sci. 2021. PMID: 34298915 Free PMC article. Review.
-
LRRTM4 is a member of the transsynaptic complex between rod photoreceptors and bipolar cells.J Comp Neurol. 2021 Jan;529(1):221-233. doi: 10.1002/cne.24944. Epub 2020 Jun 27. J Comp Neurol. 2021. PMID: 32390181 Free PMC article.
-
Extended functional rescue following AAV gene therapy in a canine model of LRIT3-congenital stationary night blindness.Vision Res. 2023 Aug;209:108260. doi: 10.1016/j.visres.2023.108260. Epub 2023 May 21. Vision Res. 2023. PMID: 37220680 Free PMC article.
-
Late gene therapy limits the restoration of retinal function in a mouse model of retinitis pigmentosa.Nat Commun. 2023 Dec 12;14(1):8256. doi: 10.1038/s41467-023-44063-8. Nat Commun. 2023. PMID: 38086857 Free PMC article.
References
-
- Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS, Gregg RG (2002) Role of the β2 subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci 43:1595–1603. - PubMed
-
- Ball SL, Pardue M, McCall M, Gregg R, Peachey N (2003) Immunohistochemical analysis of the outer plexiform layer in the Nob mouse. Invest Ophthalmol Vis Sci 44:1865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
