Dopamine-induced calcium signaling in olfactory bulb astrocytes
- PMID: 31959788
- PMCID: PMC6971274
- DOI: 10.1038/s41598-020-57462-4
Dopamine-induced calcium signaling in olfactory bulb astrocytes
Abstract
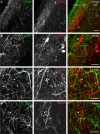
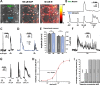
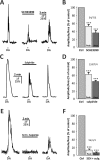
It is well established that astrocytes respond to the major neurotransmitters glutamate and GABA with cytosolic calcium rises, whereas less is known about the effect of dopamine on astroglial cells. In the present study, we used confocal calcium imaging in mouse brain slices of the olfactory bulb, a brain region with a large population of dopaminergic neurons, to investigate calcium signaling evoked by dopamine in astrocytes. Our results show that application of dopamine leads to a dose-dependent cytosolic calcium rise in astrocytes (EC50 = 76 µM) which is independent of neuronal activity and mainly mediated by PLC/IP3-dependent internal calcium release. Antagonists of both D1- and D2-class dopamine receptors partly reduce the dopaminergic calcium response, indicating that both receptor classes contribute to dopamine-induced calcium transients in olfactory bulb astrocytes.
Conflict of interest statement
The authors declare no competing interests.
Figures
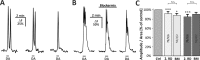

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

