Clinical heterogeneity under induction with different dosages of cytarabine in core binding factor acute myeloid leukaemia
- PMID: 31959790
- PMCID: PMC6971028
- DOI: 10.1038/s41598-020-57414-y
Clinical heterogeneity under induction with different dosages of cytarabine in core binding factor acute myeloid leukaemia
Abstract
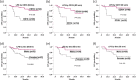
Repeated cycles of post-remission high-dose cytarabine (Ara-C) have been suggested to improve survival in core binding factor (CBF) acute myeloid leukaemia (AML). High-dose Ara-C used for induction regimens has also been reported to be associated with increased treatment-related mortality (TRM). Few data are available about intermediate-dose Ara-C serving as induction therapy. The aim of our study was to compare the tolerance and outcomes of standard- and intermediate-dose levels of Ara-C as induction in CBF AML and to analyse the clinical heterogeneity of the two AML entities under these induction settings. We retrospectively investigated the outcomes in adults with CBF AML induced with regimens based on standard-dose Ara-C at 100 to 200 mg/m2 or intermediate-dose Ara-C at 1,000 mg/m2. In total, 152 patients with t(8; 21) and 54 patients with inv(16) AML were administered an induction regimen containing anthracyclines plus either standard- or intermediate-dose Ara-C. After a single course of induction, the complete remission (CR) rate in the inv(16) cohort was 52/52 (100%), higher than the 127/147 (86.4%) in the t(8; 21) cohort (P = 0.005). Intermediate-dose Ara-C (HR = 9.931 [2.135-46.188], P = 0.003) and negative KITmut (HR = 0.304 [0.106-0.874], P = 0.027) independently produced an increased CR rate in the t(8; 21) cohort. Positive CD19 expression (HR = 0.133 [0.045-0.387], P = 0.000) and sex (male) (HR = 0.238 [0.085-0.667], P = 0.006) were associated with superior leukaemia-free survival (LFS) in the t(8; 21) cohort independently of KITmut status or the induction regimen. We conclude that intermediate-dose Ara-C is superior to standard-dose Ara-C for induction of remission in t(8; 21) AML, and CD19 status and sex independently confer prognostic significance for LFS. The KITmut status alone does not have an independent effect on survival in t(8; 21) AML. More intensive induction therapy is unnecessary in inv(16) AML.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

