Precise safety pharmacology studies of lapatinib for onco-cardiology assessed using in vivo canine models
- PMID: 31959820
- PMCID: PMC6971088
- DOI: 10.1038/s41598-020-57601-x
Precise safety pharmacology studies of lapatinib for onco-cardiology assessed using in vivo canine models
Abstract
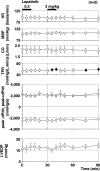
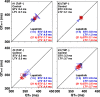
Cancer chemotherapies have improved prognosis in cancer patients, resulting in a large and rapidly increasing number of cancer survivors. "Onco-cardiology" or "cardio-oncology" is a new discipline for addressing the unanticipated cardiac side effects of newly developed cancer drugs. Lapatinib, a tyrosine kinase inhibitor suppressing the epidermal growth factor receptor and ErbB2, has been used in advanced or metastatic breast cancer treatment. Reportedly, lapatinib has induced cardiovascular adverse events including QT-interval prolongation and heart failure. However, they have not been predicted by preclinical studies. Hence, a new method to assess the tyrosine kinase inhibitor-induced adverse effects needs to be established. Here, we intravenously administered lapatinib to halothane-anaesthetised dogs, evaluating cardiohemodynamic, electrophysiological, and echocardiographic profiles for pharmacological safety assessments. We intravenously administered lapatinib to chronic atrioventricular block beagle dogs to assess its proarrhythmic potential. The therapeutic concentration of lapatinib significantly increased total peripheral vascular resistance, QT, QTc, monophasic action potential (MAP)90(sinus), MAP90(CL400), effective refractory period, and plasma concentration of cardiac troponin I (cTnI), suggesting that lapatinib prolonged the ventricular repolarization without inducing lethal ventricular arrhythmia. Careful monitoring of plasma cTnI concentration and an electrocardiogram could be supportive biomarkers, predicting the onset of lapatinib-induced cardiovascular adverse events.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- ICHS9: Nonclinical Evaluation for Anticancer Pharmaceuticals, https://www.ich.org/products/guidelines/safety/article/safety-guidelines... (Accessed 27 July 2019) (2009).
-
- ICHS7: Safety Pharmacology Studies for Human Pharmaceuticals, https://www.ich.org/products/guidelines/safety/article/safety-guidelines... (Accessed 27 July 2019) (2000).
-
- European Medicines Agency: Assessment Report for Tyverb. Doc.Ref.: EMEA/302222/2008, https://www.ema.europa.eu/en/documents/assessment-report/tyverb-epar-pub... (Accessed 27 July 2019) (2008).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

