Analysis of O-glycoforms of the IgA1 hinge region by sequential deglycosylation
- PMID: 31959827
- PMCID: PMC6971281
- DOI: 10.1038/s41598-020-57510-z
Analysis of O-glycoforms of the IgA1 hinge region by sequential deglycosylation
Erratum in
-
Author Correction: Analysis of O-glycoforms of the IgA1 hinge region by sequential deglycosylation.Sci Rep. 2021 Oct 21;11(1):21209. doi: 10.1038/s41598-021-00847-w. Sci Rep. 2021. PMID: 34675329 Free PMC article. No abstract available.
Abstract
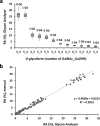
A common renal disease, immunoglobulin A (IgA) nephropathy (IgAN), is associated with glomerular deposition of IgA1-containing immune complexes. IgA1 hinge region (HR) has up to six clustered O-glycans consisting of Ser/Thr-linked N-acetylgalactosamine with β1,3-linked galactose and variable sialylation. IgA1 glycoforms with some galactose-deficient (Gd) HR O-glycans play a key role in IgAN pathogenesis. The clustered and variable O-glycans make the IgA1 glycomic analysis challenging and better approaches are needed. Here, we report a comprehensive analytical workflow for IgA1 HR O-glycoform analysis. We combined an automated quantitative analysis of the HR O-glycopeptide profiles with sequential deglycosylation to remove all but Gd O-glycans from the HR. The workflow was tested using serum IgA1 from healthy subjects. Twelve variants of glycopeptides corresponding to the HR with three to six O-glycans were detected; nine glycopeptides carried up to three Gd O-glycans. Sites with Gd O-glycans were unambiguously identified by electron-transfer/higher-energy collision dissociation tandem mass spectrometry. Extracted ion chromatograms of isomeric glycoforms enabled quantitative assignment of Gd sites. The most frequent Gd site was T236, followed by S230, T233, T228, and S232. The new workflow for quantitative profiling of IgA1 HR O-glycoforms with site-specific resolution will enable identification of pathogenic IgA1 HR O-glycoforms in IgAN.
Conflict of interest statement
M. B. Renfrow and J. Novak are co-founders and co-owners of and consultants for Reliant Glycosciences, LLC and co-inventors on the US patent application 14/318,082 (assigned to UAB Research Foundation that distributes royalties to the inventors). The other authors declare no competing interests.
Figures





References
-
- Shields RL, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcγ RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002;277:26733–26740. - PubMed
-
- Shinkawa T, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003;278:3466–3473. - PubMed
-
- Tomana M, Schrohenloher RE, Koopman WJ, Alarcon GS, Paul WA. Abnormal glycosylation of serum IgG from patients with chronic inflammatory diseases. Arthritis Rheum. 1988;31:333–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

