Fecal dysbiosis in infants with cystic fibrosis is associated with early linear growth failure
- PMID: 31959989
- PMCID: PMC7018602
- DOI: 10.1038/s41591-019-0714-x
Fecal dysbiosis in infants with cystic fibrosis is associated with early linear growth failure
Abstract
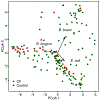
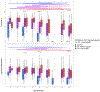
Most infants with cystic fibrosis (CF) have pancreatic exocrine insufficiency that results in nutrient malabsorption and requires oral pancreatic enzyme replacement. Newborn screening for CF has enabled earlier diagnosis, nutritional intervention and enzyme replacement for these infants, allowing most infants with CF to achieve their weight goals by 12 months of age1. Nevertheless, most infants with CF continue to have poor linear growth during their first year of life1. Although this early linear growth failure is associated with worse long-term respiratory function and survival2,3, the determinants of body length in infants with CF have not been defined. Several characteristics of the CF gastrointestinal (GI) tract, including inflammation, maldigestion and malabsorption, may promote intestinal dysbiosis4,5. As GI microbiome activities are known to affect endocrine functions6,7, the intestinal microbiome of infants with CF may also impact growth. We identified an early, progressive fecal dysbiosis that distinguished infants with CF and low length from infants with CF and normal length. This dysbiosis included altered abundances of taxa that perform functions that are important for GI health, nutrient harvest and growth hormone signaling, including decreased abundance of Bacteroidetes and increased abundance of Proteobacteria. Thus, the GI microbiota represent a potential therapeutic target for the correction of low linear growth in infants with CF.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures









References
Methods-only References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

