Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma
- PMID: 31959992
- PMCID: PMC7781235
- DOI: 10.1038/s41591-019-0737-3
Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma
Erratum in
-
Author Correction: Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma.Nat Med. 2020 May;26(5):803. doi: 10.1038/s41591-020-0864-x. Nat Med. 2020. PMID: 32291416 Free PMC article.
Abstract
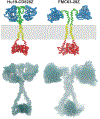
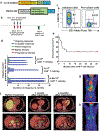
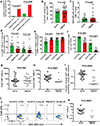
Anti-CD19 chimeric antigen receptor (CAR)-expressing T cells are an effective treatment for B-cell lymphoma, but often cause neurologic toxicity. We treated 20 patients with B-cell lymphoma on a phase I, first-in-human clinical trial of T cells expressing the new anti-CD19 CAR Hu19-CD828Z (NCT02659943). The primary objective was to assess safety and feasibility of Hu19-CD828Z T-cell therapy. Secondary objectives included assessments of blood levels of CAR T cells, anti-lymphoma activity, second infusions and immunogenicity. All objectives were met. Fifty-five percent of patients who received Hu19-CD828Z T cells obtained complete remission. Hu19-CD828Z T cells had clinical anti-lymphoma activity similar to that of T cells expressing FMC63-28Z, an anti-CD19 CAR tested previously by our group, which contains murine binding domains and is used in axicabtagene ciloleucel. However, severe neurologic toxicity occurred in only 5% of patients who received Hu19-CD828Z T cells, whereas 50% of patients who received FMC63-28Z T cells experienced this degree of toxicity (P = 0.0017). T cells expressing Hu19-CD828Z released lower levels of cytokines than T cells expressing FMC63-28Z. Lower levels of cytokines were detected in blood from patients who received Hu19-CD828Z T cells than in blood from those who received FMC63-28Z T cells, which could explain the lower level of neurologic toxicity associated with Hu19-CD828Z. Levels of cytokines released by CAR-expressing T cells particularly depended on the hinge and transmembrane domains included in the CAR design.
Conflict of interest statement
Competing Interests
This work was supported by intramural funding of the Center for Cancer Research, National Cancer Institute, NIH. In addition, the NCI has Cooperative Research and Development Agreements with Kite Pharma, a Gilead Company that support development of anti-CD19 CAR T-cell therapies, and both James N. Kochenderfer and Steven A. Rosenberg are NCI Principle Investigators of these Research Agreements. James Kochenderfer has a patent application for the Hu19-CD828Z CAR and has received royalty payments from Kite, a Gilead Company. Adrian Bot, John Rossi, Allen Xue, and Nathalie Scholler are all employees of Kite, a Gilead Company.
Figures









Comment in
-
Human Binding Domains Reduce Neurotoxicity of Anti-CD19 CAR-T Cells.Cancer Discov. 2020 Mar;10(3):338. doi: 10.1158/2159-8290.CD-RW2020-017. Epub 2020 Jan 31. Cancer Discov. 2020. PMID: 32005671
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

