Clinical Considerations When Initiating and Titrating Insulin Degludec/Liraglutide (IDegLira) in People with Type 2 Diabetes
- PMID: 31960258
- PMCID: PMC7007423
- DOI: 10.1007/s40265-019-01245-3
Clinical Considerations When Initiating and Titrating Insulin Degludec/Liraglutide (IDegLira) in People with Type 2 Diabetes
Abstract
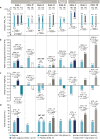
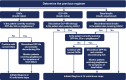
Therapeutic inertia is a substantial obstacle to the initiation of insulin therapy in people with uncontrolled type 2 diabetes (T2D). This effect has in part been perpetuated by concerns over the impact of a burdensome regimen and the increased risk of hypoglycemia and body weight gain often associated with insulin use. An effective, yet simple, less burdensome regimen with a lower risk of body weight gain and hypoglycemia compared with an insulin-only regimen, may help to address these concerns more effectively. We review the available clinical and real-world data on IDegLira, a once-daily, injectable, fixed-ratio combination of insulin degludec (degludec) and the glucagon-like peptide-1 receptor agonist (GLP-1RA) liraglutide, in people with T2D. Evidence from the comprehensive DUAL clinical trial program suggests an advantage of IDegLira over traditional insulin therapies in a number of clinical outcomes, including maintenance of glycemic control, achievement of glycemic targets, reducing the risk of hypoglycemia, and body weight loss. These findings were demonstrated in participants with T2D irrespective of prior GLP-1RA and insulin use. Furthermore, the individual components of IDegLira have confirmed safety (degludec) or significant benefit in terms of improvement of cardiovascular risk (liraglutide). As an injectable therapy that is simple to titrate, IDegLira has the potential to optimize the ability to achieve relevant glycemic targets, and offers a suitable treatment option for people with T2D requiring insulin therapy who are at risk of hypoglycemia or weight gain.
Conflict of interest statement
Stewart Harris has received fees as a consultant for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Medtronic, Merck & Co, Novo Nordisk, and Sanofi; for research support for the Juvenile Diabetes Research Foundation, the Canadian Institutes of Health Research, the Lawson Foundation, Novo Nordisk, Sanofi, AstraZeneca, and Health Canada/First Nations and Inuit Health Branch; and as an advisory board member for the Juvenile Diabetes Research Foundation, the Canadian Institutes of Health Research, the Lawson Foundation, Novo Nordisk, Sanofi, AstraZeneca, and Health Canada/First Nations and Inuit Health Branch. Martin J. Abrahamson has received consulting fees or honoraria from Novo Nordisk and Web MD Health Services. Antonio Ceriello has been an advisory board member for AstraZeneca, Boehringer Ingelheim, DOC Generici, Eli Lilly, Janssen, Mundipharma, Novo Nordisk, and OM Pharma; given lectures for Boehringer Ingelheim, Eli Lilly, Mundipharma, Novartis, Novo Nordisk, Sanofi, and Takeda; and received research grants from AstraZeneca, Eli Lilly, Mitsubishi, and Novartis. Marc Evans has received consulting fees or honoraria from Novo Nordisk, NAPP, MSD, AstraZeneca, Sunovion, and Novartis; and lecture/speaker bureau fees from Novo Nordisk, NAPP, Mundipharma, AstraZeneca, MSD, Sunovion, and Novartis. Roger Lehmann has received honoraria for advisory boards from Eli Lilly, Sanofi, MSD, Novo Nordisk, Roche, Medtronic, and Boehringer-Ingelheim; and fees for lecturing, consultancy work, and attendance at conferences from AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk, and Sanofi. Guillaume Charpentier has received consulting and lecture/speaker bureau fees from Novo Nordisk. Richard Holt has received grants from Novo Nordisk as an investigator on clinical trials; consulting fees or honorarium from Mylan and Novo Nordisk; payment for lectures/speaker’s bureau from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Lundbeck, Novo Nordisk, Otsuka, Sanofi, and Sunovion; and royalties from Wiley as editor-in-chief of Diabetic Medicine and the 5th Edition of the Textbook of Diabetes. Nóra Hosszúfalusi has received consulting fees or honoraria from AstraZeneca, Eli Lilly, and Novo Nordisk; support for travel from Novo Nordisk and 77 Elektronika Hungary; and payment for lectures/speaker’s bureaus from Novo Nordisk, Eli Lilly, AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, and Sanofi Aventis. Sultan Linjawi received payment for lectures/speaker’s bureau for Novartis. Guy Rutten has received fees as a member of a global advisory board from Novo Nordisk. Andreas Liebl has received fees for consulting from Boehringer Ingelheim, DexCom, Lilly, Medtronic, MSD, Novo Nordisk, and Roche; travel support from Boehringer Ingelheim, Novo Nordisk, and Sanofi; and payment for lectures/speaker’s bureau from AstraZeneca, Bayer, Becton Dickinson, Boehringer Ingelheim, Bristol Myers Squibb, DexCom, Medtronic, MSD, Novo Nordisk, OmniaMed, Roche, and Sanofi. Tina Vilsbøll declares personal fees from Amgen, Boehringer Ingelheim, Eli Lilly, AstraZeneca, Merck Sharp & Dohme, Sanofi, Sun Pharmaceuticals, Novo Nordisk, and Bristol-Myers Squibb, and grants (to her institution) from Eli Lilly, Boehringer Ingelheim, and Novo Nordisk.
Figures

References
-
- Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46(1):3–19. - PubMed
-
- American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes 2018. Diabetes Care. 2019;42(Suppl 1):S90–S102. - PubMed
-
- Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41(12):2669–2701. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

