Shifting equilibriums in Alzheimer's disease: the complex roles of microglia in neuroinflammation, neuronal survival and neurogenesis
- PMID: 31960800
- PMCID: PMC7047786
- DOI: 10.4103/1673-5374.272571
Shifting equilibriums in Alzheimer's disease: the complex roles of microglia in neuroinflammation, neuronal survival and neurogenesis
Abstract
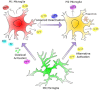
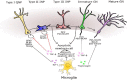
Alzheimer's disease is the leading cause of dementia. Its increased prevalence in developed countries, due to the sharp rise in ageing populations, presents one of the costliest challenges to modern medicine. In order to find disease-modifying therapies to confront this challenge, a more complete understanding of the pathogenesis of Alzheimer's disease is necessary. Recent studies have revealed increasing evidence for the roles played by microglia, the resident innate immune system cells of the brain. Reflecting the well-established roles of microglia in reacting to pathogens and inflammatory stimuli, there is now a growing literature describing both protective and detrimental effects for individual cytokines and chemokines produced by microglia in Alzheimer's disease. A smaller but increasing number of studies have also addressed the divergent roles played by microglial neurotrophic and neurogenic factors, and how their perturbation may play a key role in the pathogenesis of Alzheimer's disease. Here we review recent findings on the roles played by microglia in neuroinflammation, neuronal survival and neurogenesis in Alzheimer's disease. In each case, landmark studies have provided evidence for the divergent ways in which microglia can either promote neuronal function and survival, or perturb neuronal function, leading to cell death. In many cases, the secreted molecules of microglia can lead to divergent effects depending on the magnitude and context of microglial activation. This suggests that microglial functions must be maintained in a fine equilibrium, in order to support healthy neuronal function, and that the cellular microenvironment in the Alzheimer's disease brain disrupts this fine balance, leading to neurodegeneration. Thus, an understanding of microglial homeostasis, both in health and across the trajectory of the disease state, will improve our understanding of the pathogenic mechanisms underlying Alzheimer's disease, and will hopefully lead to the development of microglial-based therapeutic strategies to restore equilibrium in the Alzheimer's disease brain.
Keywords: Alzheimer’s disease; IGF-1; Trem2; adult neurogenesis; microglia; neuroinflammation.
Conflict of interest statement
None
Figures

References
-
- Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An english translation of alzheimer’s 1907 paper, “über eine eigenartige erkankung der hirnrinde”. Clin Anat. 1995;8:429–431. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

