Sensory Nociceptive Neurons Contribute to Host Protection During Enteric Infection With Citrobacter rodentium
- PMID: 31960920
- PMCID: PMC7289549
- DOI: 10.1093/infdis/jiaa014
Sensory Nociceptive Neurons Contribute to Host Protection During Enteric Infection With Citrobacter rodentium
Abstract
Background: Neurons are an integral component of the immune system that functions to coordinate responses to bacterial pathogens. Sensory nociceptive neurons that can detect bacterial pathogens are found throughout the body with dense innervation of the intestinal tract.
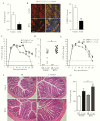
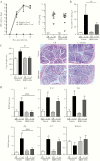
Methods: In this study, we assessed the role of these nerves in the coordination of host defenses to Citrobacter rodentium. Selective ablation of nociceptive neurons significantly increased bacterial burden 10 days postinfection and delayed pathogen clearance.
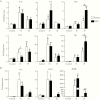
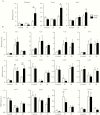
Results: Because the sensory neuropeptide CGRP (calcitonin gene-related peptide) regulates host responses during infection of the skin, lung, and small intestine, we assessed the role of CGRP receptor signaling during C rodentium infection. Although CGRP receptor blockade reduced certain proinflammatory gene expression, bacterial burden and Il-22 expression was unaffected.
Conclusions: Our data highlight that sensory nociceptive neurons exert a significant host protective role during C rodentium infection, independent of CGRP receptor signaling.
Keywords: Citrobacter rodentium; CGRP; TRPV1; nociceptors.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

References
-
- Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther 2010; 125:181–95. - PubMed

