Leaf growth in early development is key to biomass heterosis in Arabidopsis
- PMID: 31960925
- PMCID: PMC7178430
- DOI: 10.1093/jxb/eraa006
Leaf growth in early development is key to biomass heterosis in Arabidopsis
Abstract
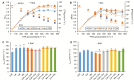
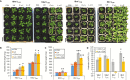
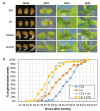
Arabidopsis thaliana hybrids have similar properties to hybrid crops, with greater biomass relative to the parents. We asked whether the greater biomass was due to increased photosynthetic efficiency per unit leaf area or to overall increased leaf area and increased total photosynthate per plant. We found that photosynthetic parameters (electron transport rate, CO2 assimilation rate, chlorophyll content, and chloroplast number) were unchanged on a leaf unit area and unit fresh weight basis between parents and hybrids, indicating that heterosis is not a result of increased photosynthetic efficiency. To investigate the possibility of increased leaf area producing more photosynthate per plant, we studied C24×Landsberg erecta (Ler) hybrids in detail. These hybrids have earlier germination and leaf growth than the parents, leading to a larger leaf area at any point in development of the plant. The developing leaves of the hybrids are significantly larger than those of the parents, with consequent greater production of photosynthate and an increased contribution to heterosis. The set of leaves contributing to heterosis changes as the plant develops; the four most recently emerged leaves make the greatest contribution. As a leaf matures, its contribution to heterosis attenuates. While photosynthesis per unit leaf area is unchanged at any stage of development in the hybrid, leaf area is greater and the amount of photosynthate per plant is increased.
Keywords: Arabidopsis; CO2 assimilation; biomass vigour; early germination; electron transport rate; heterosis; hybrid; leaf development; photosynthesis.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures


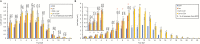
References
-
- Baig MJ, Swain P, Murty KS. 1998. The photosynthetic efficiency of some elite rice hybrids and restorers. Photosynthetica 35, 241–246.
-
- Demmig B, Björkman O. 1987. Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 171, 171–184. - PubMed
-
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG. 1999. Cell cycling and cell enlargement in developing leaves of Arabidopsis. Developmental Biology 215, 407–419. - PubMed
-
- Edwards GE, Baker NR. 1993. Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynthesis Research 37, 89–102. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

