Characterization of Alzheimer Disease Biomarker Discrepancies Using Cerebrospinal Fluid Phosphorylated Tau and AV1451 Positron Emission Tomography
- PMID: 31961372
- PMCID: PMC6990861
- DOI: 10.1001/jamaneurol.2019.4749
Characterization of Alzheimer Disease Biomarker Discrepancies Using Cerebrospinal Fluid Phosphorylated Tau and AV1451 Positron Emission Tomography
Erratum in
-
Errors in Text, Figures, and Author Affiliation.JAMA Neurol. 2020 Apr 1;77(4):527. doi: 10.1001/jamaneurol.2020.0044. JAMA Neurol. 2020. PMID: 32065607 Free PMC article. No abstract available.
-
Omission of Group Information.JAMA Neurol. 2021 Mar 1;78(3):370. doi: 10.1001/jamaneurol.2020.5322. JAMA Neurol. 2021. PMID: 33492331 Free PMC article. No abstract available.
Abstract
Importance: Fluid and imaging biomarkers of Alzheimer disease (AD) are often used interchangeably, but some biomarkers may reveal earlier stages of disease.
Objective: To characterize individuals with tau abnormality indicated by cerebrospinal fluid (CSF) assay or positron emission tomography (PET).
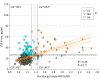
Design, setting, and participants: Between 2010 and 2019, 322 participants in the Alzheimer's Disease Neuroimaging Initiative (ADNI) underwent CSF and PET assessments of tau pathology. Data-driven, clinically relevant thresholds for CSF phosphorylated tau (P-tau) (≥26.64 pg/mL) and flortaucipir-PET meta-regions of interest (ROI) (standard uptake value ratio ≥1.37) indicated participants' tau status as CSF-/PET-, CSF+/PET-, CSF-/PET+, and CSF+/PET+. Of 1659 ADNI participants with a CSF or flortaucipir assessment, 588 had both measures (1071 were excluded). Among these, 266 were further excluded because they did not have flortaucipir and CSF testing within less than 25 months, leaving 322 for analysis. Of these, 213 were cognitively unimpaired (CU); 98 had mild cognitive impairment (MCI); and 11 had AD dementia.
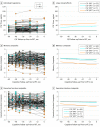
Main outcomes and measures: We compared tau-positive vs tau-negative groups as indicated by either modality or demographic and clinical variables, amyloid β-PET burden, and flortaucipir-PET binding across Braak stage-related ROIs. We also compared 5-year rates of CSF P-tau accumulation and cognitive decline prior to flortaucipir-PET scanning.
Results: Among the 322 study participants, 180 were women (56%), and the mean (SD) age was 73.08 (7.37) years. Two hundred ten participants were CSF-/PET- (65%); 63 were CSF+/PET- (19.5%); 15 were CSF-/PET+ (4.6%); and 34 were CSF+/PET+ (10.5%). Most CSF-/PET+ participants had measures near CSF or PET tau thresholds. The CSF+/PET- participants showed faster 5-year accrual of P-tau and increased flortaucipir-PET binding in early Braak ROIs but similar memory decline compared with CSF-/PET- participants. Tau-positive individuals by either measure showed increased amyloid β-PET burden. All CSF+/PET+ individuals were amyloid-positive, and 26 had MCI or AD dementia (76%). Compared with the CSF-/PET- group, CSF+/PET+ individuals had experienced faster 5-year accrual of CSF P-tau and decline in memory and executive function, resulting in reduced cognitive abilities at the time of flortaucipir-PET assessment.
Conclusions and relevance: Suprathreshold CSF P-tau without flortaucipir-PET abnormality may indicate a stage of AD development characterized by early tau abnormality without measurable loss in cognitive performance. Persons with both tau CSF and PET abnormality appear to have reduced cognitive capacities resulting from faster antecedent cognitive decline. Elevation of CSF P-tau appears to precede flortaucipir-PET positivity in the progression of AD pathogenesis and related cognitive decline.
Conflict of interest statement
Figures

Similar articles
-
18F-Flortaucipir PET Associations with Cerebrospinal Fluid, Cognition, and Neuroimaging in Mild Cognitive Impairment due to Alzheimer's Disease.J Alzheimers Dis. 2020;74(2):589-601. doi: 10.3233/JAD-191330. J Alzheimers Dis. 2020. PMID: 32065800 Clinical Trial.
-
Characterization of Alzheimer's tau biomarker discordance using plasma, CSF, and PET.Alzheimers Res Ther. 2021 May 4;13(1):93. doi: 10.1186/s13195-021-00834-3. Alzheimers Res Ther. 2021. PMID: 33947453 Free PMC article.
-
Association of Initial β-Amyloid Levels With Subsequent Flortaucipir Positron Emission Tomography Changes in Persons Without Cognitive Impairment.JAMA Neurol. 2021 Feb 1;78(2):217-228. doi: 10.1001/jamaneurol.2020.3921. JAMA Neurol. 2021. PMID: 33074304 Free PMC article.
-
2014 Update of the Alzheimer's Disease Neuroimaging Initiative: A review of papers published since its inception.Alzheimers Dement. 2015 Jun;11(6):e1-120. doi: 10.1016/j.jalz.2014.11.001. Alzheimers Dement. 2015. PMID: 26073027 Free PMC article. Review.
-
What's the cut-point?: a systematic investigation of tau PET thresholding methods.Alzheimers Res Ther. 2022 Apr 5;14(1):49. doi: 10.1186/s13195-022-00986-w. Alzheimers Res Ther. 2022. PMID: 35382866 Free PMC article. Review.
Cited by
-
Discordant Alzheimer's neurodegenerative biomarkers and their clinical outcomes.Ann Clin Transl Neurol. 2020 Oct;7(10):1996-2009. doi: 10.1002/acn3.51196. Epub 2020 Sep 19. Ann Clin Transl Neurol. 2020. PMID: 32949193 Free PMC article.
-
Errors in Text, Figures, and Author Affiliation.JAMA Neurol. 2020 Apr 1;77(4):527. doi: 10.1001/jamaneurol.2020.0044. JAMA Neurol. 2020. PMID: 32065607 Free PMC article. No abstract available.
-
Unsupervised [18F]Flortaucipir cutoffs for tau positivity and staging in Alzheimer's disease.Eur J Nucl Med Mol Imaging. 2023 Sep;50(11):3265-3275. doi: 10.1007/s00259-023-06280-7. Epub 2023 Jun 5. Eur J Nucl Med Mol Imaging. 2023. PMID: 37272955 Free PMC article.
-
Hippocampal Subfield Volume in Relation to Cerebrospinal Fluid Amyloid-ß in Early Alzheimer's Disease: Diagnostic Utility of 7T MRI.Eur J Neurol. 2025 Feb;32(2):e70076. doi: 10.1111/ene.70076. Eur J Neurol. 2025. PMID: 39921301 Free PMC article.
-
Effect of APOE ε4 genotype on amyloid-β, glucose metabolism, and gray matter volume in cognitively normal individuals and amnestic mild cognitive impairment.Eur J Neurol. 2023 Mar;30(3):587-596. doi: 10.1111/ene.15656. Epub 2022 Dec 29. Eur J Neurol. 2023. PMID: 36448771 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

