Effect of Hydroxyethyl Starch vs Saline for Volume Replacement Therapy on Death or Postoperative Complications Among High-Risk Patients Undergoing Major Abdominal Surgery: The FLASH Randomized Clinical Trial
- PMID: 31961418
- PMCID: PMC6990683
- DOI: 10.1001/jama.2019.20833
Effect of Hydroxyethyl Starch vs Saline for Volume Replacement Therapy on Death or Postoperative Complications Among High-Risk Patients Undergoing Major Abdominal Surgery: The FLASH Randomized Clinical Trial
Abstract
Importance: It is not known if use of colloid solutions containing hydroxyethyl starch (HES) to correct for intravascular deficits in high-risk surgical patients is either effective or safe.
Objective: To evaluate the effect of HES 130/0.4 compared with 0.9% saline for intravascular volume expansion on mortality and postoperative complications after major abdominal surgery.
Design, setting, and participants: Multicenter, double-blind, parallel-group, randomized clinical trial of 775 adult patients at increased risk of postoperative kidney injury undergoing major abdominal surgery at 20 university hospitals in France from February 2016 to July 2018; final follow-up was in October 2018.
Interventions: Patients were randomized to receive fluid containing either 6% HES 130/0.4 diluted in 0.9% saline (n = 389) or 0.9% saline alone (n = 386) in 250-mL boluses using an individualized hemodynamic algorithm during surgery and for up to 24 hours on the first postoperative day, defined as ending at 7:59 am the following day.
Main outcomes and measures: The primary outcome was a composite of death or major postoperative complications at 14 days after surgery. Secondary outcomes included predefined postoperative complications within 14 days after surgery, durations of intensive care unit and hospital stays, and all-cause mortality at postoperative days 28 and 90.
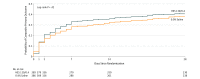
Results: Among 826 patients enrolled (mean age, 68 [SD, 7] years; 91 women [12%]), 775 (94%) completed the trial. The primary outcome occurred in 139 of 389 patients (36%) in the HES group and 125 of 386 patients (32%) in the saline group (difference, 3.3% [95% CI, -3.3% to 10.0%]; relative risk, 1.10 [95% CI, 0.91-1.34]; P = .33). Among 12 prespecified secondary outcomes reported, 11 showed no significant difference, but a statistically significant difference was found in median volume of study fluid administered on day 1: 1250 mL (interquartile range, 750-2000 mL) in the HES group and 1500 mL (interquartile range, 750-2150 mL) in the saline group (median difference, 250 mL [95% CI, 83-417 mL]; P = .006). At 28 days after surgery, 4.1% and 2.3% of patients had died in the HES and saline groups, respectively (difference, 1.8% [95% CI, -0.7% to 4.3%]; relative risk, 1.76 [95% CI, 0.79-3.94]; P = .17).
Conclusions and relevance: Among patients at risk of postoperative kidney injury undergoing major abdominal surgery, use of HES for volume replacement therapy compared with 0.9% saline resulted in no significant difference in a composite outcome of death or major postoperative complications within 14 days after surgery. These findings do not support the use of HES for volume replacement therapy in such patients.
Trial registration: ClinicalTrials.gov Identifier: NCT02502773.
Conflict of interest statement
Figures

Comment in
-
Hydroxyethyl Starch for Fluid Replacement Therapy in High-Risk Surgical Patients: Context and Caution.JAMA. 2020 Jan 21;323(3):217-218. doi: 10.1001/jama.2019.20141. JAMA. 2020. PMID: 31961399 No abstract available.
-
Perioperative goal-directed fluid optimisation: Is there still a place for hydroxyethyl starch in 2020?Anaesth Crit Care Pain Med. 2020 Apr;39(2):185-186. doi: 10.1016/j.accpm.2020.03.011. Epub 2020 Mar 20. Anaesth Crit Care Pain Med. 2020. PMID: 32201194 No abstract available.
-
Hydroxyethyl Starch vs Saline for Volume Expansion After Abdominal Surgery.JAMA. 2020 Jul 14;324(2):199. doi: 10.1001/jama.2020.6971. JAMA. 2020. PMID: 32662855 No abstract available.
-
Hydroxyethyl Starch vs Saline for Volume Expansion After Abdominal Surgery.JAMA. 2020 Jul 14;324(2):200. doi: 10.1001/jama.2020.6974. JAMA. 2020. PMID: 32662856 No abstract available.
-
Hydroxyethyl Starch vs Saline for Volume Expansion After Abdominal Surgery.JAMA. 2020 Jul 14;324(2):199-200. doi: 10.1001/jama.2020.6977. JAMA. 2020. PMID: 32662857 No abstract available.
-
Hydroxyethyl Starch vs Saline for Volume Expansion After Abdominal Surgery.JAMA. 2020 Jul 14;324(2):199. doi: 10.1001/jama.2020.6980. JAMA. 2020. PMID: 32662858 No abstract available.
References
-
- Myles PS, Bellomo R, Corcoran T, et al. ; Australian and New Zealand College of Anaesthetists Clinical Trials Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group . Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378(24):2263-2274. doi: 10.1056/NEJMoa1801601 - DOI - PubMed
-
- Annane D, Siami S, Jaber S, et al. ; CRISTAL Investigators . Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310(17):1809-1817. doi: 10.1001/jama.2013.280502 - DOI - PubMed

