Effect of Early Surgery vs Endoscopy-First Approach on Pain in Patients With Chronic Pancreatitis: The ESCAPE Randomized Clinical Trial
- PMID: 31961419
- PMCID: PMC6990680
- DOI: 10.1001/jama.2019.20967
Effect of Early Surgery vs Endoscopy-First Approach on Pain in Patients With Chronic Pancreatitis: The ESCAPE Randomized Clinical Trial
Abstract
Importance: For patients with painful chronic pancreatitis, surgical treatment is postponed until medical and endoscopic treatment have failed. Observational studies have suggested that earlier surgery could mitigate disease progression, providing better pain control and preserving pancreatic function.
Objective: To determine whether early surgery is more effective than the endoscopy-first approach in terms of clinical outcomes.
Design, setting, and participants: The ESCAPE trial was an unblinded, multicenter, randomized clinical superiority trial involving 30 Dutch hospitals participating in the Dutch Pancreatitis Study Group. From April 2011 until September 2016, a total of 88 patients with chronic pancreatitis, a dilated main pancreatic duct, and who only recently started using prescribed opioids for severe pain (strong opioids for ≤2 months or weak opioids for ≤6 months) were included. The 18-month follow-up period ended in March 2018.
Interventions: There were 44 patients randomized to the early surgery group who underwent pancreatic drainage surgery within 6 weeks after randomization and 44 patients randomized to the endoscopy-first approach group who underwent medical treatment, endoscopy including lithotripsy if needed, and surgery if needed.
Main outcomes and measures: The primary outcome was pain, measured on the Izbicki pain score and integrated over 18 months (range, 0-100 [increasing score indicates more pain severity]). Secondary outcomes were pain relief at the end of follow-up; number of interventions, complications, hospital admissions; pancreatic function; quality of life (measured on the 36-Item Short Form Health Survey [SF-36]); and mortality.
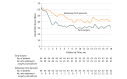
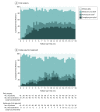
Results: Among 88 patients who were randomized (mean age, 52 years; 21 (24%) women), 85 (97%) completed the trial. During 18 months of follow-up, patients in the early surgery group had a lower Izbicki pain score than patients in the group randomized to receive the endoscopy-first approach group (37 vs 49; between-group difference, -12 points [95% CI, -22 to -2]; P = .02). Complete or partial pain relief at end of follow-up was achieved in 23 of 40 patients (58%) in the early surgery vs 16 of 41 (39%)in the endoscopy-first approach group (P = .10). The total number of interventions was lower in the early surgery group (median, 1 vs 3; P < .001). Treatment complications (27% vs 25%), mortality (0% vs 0%), hospital admissions, pancreatic function, and quality of life were not significantly different between early surgery and the endoscopy-first approach.
Conclusions and relevance: Among patients with chronic pancreatitis, early surgery compared with an endoscopy-first approach resulted in lower pain scores when integrated over 18 months. However, further research is needed to assess persistence of differences over time and to replicate the study findings.
Trial registration: ISRCTN Identifier: ISRCTN45877994.
Conflict of interest statement
Figures
Comment in
-
Tracing the Evidence to Address Painful Chronic Pancreatitis With Surgery.JAMA. 2020 Jan 21;323(3):219-220. doi: 10.1001/jama.2019.19988. JAMA. 2020. PMID: 31961398 No abstract available.
-
Surgery vs Endoscopy for Early Treatment of Chronic Pancreatitis.JAMA. 2020 Jun 2;323(21):2202. doi: 10.1001/jama.2020.4823. JAMA. 2020. PMID: 32484527 No abstract available.
References
-
- Drewes AM, Bouwense SAW, Campbell CM, et al. ; Working group for the International (IAP–APA–JPS–EPC) Consensus Guidelines for Chronic Pancreatitis . Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology. 2017;17(5):720-731. doi: 10.1016/j.pan.2017.07.006 - DOI - PubMed
-
- Löhr JM, Dominguez-Munoz E, Rosendahl J, et al. ; HaPanEU/UEG Working Group . United European gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J. 2017;5(2):153-199. doi: 10.1177/2050640616684695 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

