Effect of Renal Denervation and Catheter Ablation vs Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hypertension: The ERADICATE-AF Randomized Clinical Trial
- PMID: 31961420
- PMCID: PMC6990678
- DOI: 10.1001/jama.2019.21187
Effect of Renal Denervation and Catheter Ablation vs Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hypertension: The ERADICATE-AF Randomized Clinical Trial
Erratum in
-
Incorrect Wording.JAMA. 2020 Mar 3;323(9):896. doi: 10.1001/jama.2020.1367. JAMA. 2020. PMID: 32125382 Free PMC article. No abstract available.
Abstract
Importance: Renal denervation can reduce cardiac sympathetic activity that may result in an antiarrhythmic effect on atrial fibrillation.
Objective: To determine whether renal denervation when added to pulmonary vein isolation enhances long-term antiarrhythmic efficacy.
Design, setting, and participants: The Evaluate Renal Denervation in Addition to Catheter Ablation to Eliminate Atrial Fibrillation (ERADICATE-AF) trial was an investigator-initiated, multicenter, single-blind, randomized clinical trial conducted at 5 referral centers for catheter ablation of atrial fibrillation in the Russian Federation, Poland, and Germany. A total of 302 patients with hypertension despite taking at least 1 antihypertensive medication, paroxysmal atrial fibrillation, and plans for ablation were enrolled from April 2013 to March 2018. Follow-up concluded in March 2019.
Interventions: Patients were randomized to either pulmonary vein isolation alone (n = 148) or pulmonary vein isolation plus renal denervation (n = 154). Complete pulmonary vein isolation to v an end point of elimination of all pulmonary vein potentials; renal denervation using an irrigated-tip ablation catheter delivering radiofrequency energy to discrete sites in a spiral pattern from distal to proximal in both renal arteries.
Main outcomes and measures: The primary end point was freedom from atrial fibrillation, atrial flutter, or atrial tachycardia at 12 months. Secondary end points included procedural complications within 30 days and blood pressure control at 6 and 12 months.
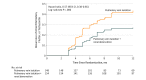
Results: Of the 302 randomized patients (median age, 60 years [interquartile range, 55-65 years]; 182 men [60.3%]), 283 (93.7%) completed the trial. All successfully underwent their assigned procedures. Freedom from atrial fibrillation, flutter, or tachycardia at 12 months was observed in 84 of 148 (56.5%) of those undergoing pulmonary vein isolation alone and in 111 of 154 (72.1%) of those undergoing pulmonary vein isolation plus renal denervation (hazard ratio, 0.57; 95% CI, 0.38 to 0.85; P = .006). Of 5 prespecified secondary end points, 4 are reported and 3 differed between groups. Mean systolic blood pressure from baseline to 12 months decreased from 151 mm Hg to 147 mm Hg in the isolation-only group and from 150 mm Hg to 135 mm Hg in the renal denervation group (between-group difference, -13 mm Hg; 95% CI, -15 to -11 mm Hg; P < .001). Procedural complications occurred in 7 patients (4.7%) in the isolation-only group and 7 (4.5%) of the renal denervation group.
Conclusions and relevance: Among patients with paroxysmal atrial fibrillation and hypertension, renal denervation added to catheter ablation, compared with catheter ablation alone, significantly increased the likelihood of freedom from atrial fibrillation at 12 months. The lack of a formal sham-control renal denervation procedure should be considered in interpreting the results of this trial.
Trial registration: ClinicalTrials.gov Identifier: NCT01873352.
Conflict of interest statement
Figures

Comment in
-
Improving Outcomes in Patients With Atrial Fibrillation and Hypertension.JAMA. 2020 Jan 21;323(3):221-222. doi: 10.1001/jama.2019.20144. JAMA. 2020. PMID: 31961401 No abstract available.
References
-
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators . Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376(9756):1903-1909. doi: 10.1016/S0140-6736(10)62039-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

