Steroid resistance in Diamond Blackfan anemia associates with p57Kip2 dysregulation in erythroid progenitors
- PMID: 31961825
- PMCID: PMC7108903
- DOI: 10.1172/JCI132284
Steroid resistance in Diamond Blackfan anemia associates with p57Kip2 dysregulation in erythroid progenitors
Abstract
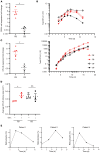
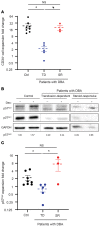
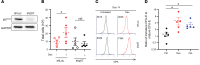
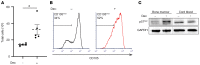
Despite the effective clinical use of steroids for the treatment of Diamond Blackfan anemia (DBA), the mechanisms through which glucocorticoids regulate human erythropoiesis remain poorly understood. We report that the sensitivity of erythroid differentiation to dexamethasone is dependent on the developmental origin of human CD34+ progenitor cells, specifically increasing the expansion of CD34+ progenitors from peripheral blood (PB) but not cord blood (CB). Dexamethasone treatment of erythroid-differentiated PB, but not CB, CD34+ progenitors resulted in the expansion of a newly defined CD34+CD36+CD71hiCD105med immature colony-forming unit-erythroid (CFU-E) population. Furthermore, proteomics analyses revealed the induction of distinct proteins in dexamethasone-treated PB and CB erythroid progenitors. Dexamethasone treatment of PB progenitors resulted in the specific upregulation of p57Kip2, a Cip/Kip cyclin-dependent kinase inhibitor, and we identified this induction as critical; shRNA-mediated downregulation of p57Kip2, but not the related p27Kip1, significantly attenuated the impact of dexamethasone on erythroid differentiation and inhibited the expansion of the immature CFU-E subset. Notably, in the context of DBA, we found that steroid resistance was associated with dysregulated p57Kip2 expression. Altogether, these data identify a unique glucocorticoid-responsive human erythroid progenitor and provide new insights into glucocorticoid-based therapeutic strategies for the treatment of patients with DBA.
Keywords: Bone marrow differentiation; Cell cycle; Development; Hematology; Hematopoietic stem cells.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

