Cystic fibrosis transmembrane conductance regulator dysfunction in platelets drives lung hyperinflammation
- PMID: 31961827
- PMCID: PMC7108932
- DOI: 10.1172/JCI129635
Cystic fibrosis transmembrane conductance regulator dysfunction in platelets drives lung hyperinflammation
Abstract
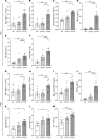
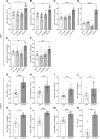
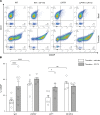
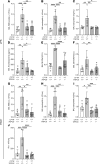
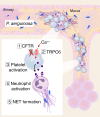
Cystic fibrosis (CF) lung disease is characterized by an inflammatory response that can lead to terminal respiratory failure. The cystic fibrosis transmembrane conductance regulator (CFTR) is mutated in CF, and we hypothesized that dysfunctional CFTR in platelets, which are key participants in immune responses, is a central determinant of CF inflammation. We found that deletion of CFTR in platelets produced exaggerated acute lung inflammation and platelet activation after intratracheal LPS or Pseudomonas aeruginosa challenge. CFTR loss of function in mouse or human platelets resulted in agonist-induced hyperactivation and increased calcium entry into platelets. Inhibition of the transient receptor potential cation channel 6 (TRPC6) reduced platelet activation and calcium flux, and reduced lung injury in CF mice after intratracheal LPS or Pseudomonas aeruginosa challenge. CF subjects receiving CFTR modulator therapy showed partial restoration of CFTR function in platelets, which may be a convenient approach to monitoring biological responses to CFTR modulators. We conclude that CFTR dysfunction in platelets produces aberrant TRPC6-dependent platelet activation, which is a major driver of CF lung inflammation and impaired bacterial clearance. Platelets and TRPC6 are what we believe to be novel therapeutic targets in the treatment of CF lung disease.
Keywords: Inflammation; Neutrophils; Platelets; Pulmonology.
Conflict of interest statement
Figures


Comment in
-
Platelets: inflammatory effector cells in the conflagration of cystic fibrosis lung disease.J Clin Invest. 2020 Apr 1;130(4):1632-1634. doi: 10.1172/JCI135949. J Clin Invest. 2020. PMID: 32175918 Free PMC article.
References
-
- Cystic Fibrosis Foundation. Patient Registry Annual Data Report 2017. Bethesda, Maryland, USA: Cystic Fibrosis Foundation; 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

