Agricultural and geographic factors shaped the North American 2015 highly pathogenic avian influenza H5N2 outbreak
- PMID: 31961906
- PMCID: PMC7004387
- DOI: 10.1371/journal.ppat.1007857
Agricultural and geographic factors shaped the North American 2015 highly pathogenic avian influenza H5N2 outbreak
Abstract
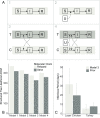
The 2014-2015 highly pathogenic avian influenza (HPAI) H5NX outbreak represents the largest and most expensive HPAI outbreak in the United States to date. Despite extensive traditional and molecular epidemiological studies, factors associated with the spread of HPAI among midwestern poultry premises remain unclear. To better understand the dynamics of this outbreak, 182 full genome HPAI H5N2 sequences isolated from commercial layer chicken and turkey production premises were analyzed using evolutionary models able to accommodate epidemiological and geographic information. Epidemiological compartmental models embedded in a phylogenetic framework provided evidence that poultry type acted as a barrier to the transmission of virus among midwestern poultry farms. Furthermore, after initial introduction, the propagation of HPAI cases was self-sustainable within the commercial poultry industries. Discrete trait diffusion models indicated that within state viral transitions occurred more frequently than inter-state transitions. Distance and sample size were very strongly supported as associated with viral transition between county groups (Bayes Factor > 30.0). Together these findings indicate that the different types of midwestern poultry industries were not a single homogenous population, but rather, the outbreak was shaped by poultry industries and geographic factors.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

