Enantioselective Synthesis of 1,12-Disubstituted [4]Helicenes
- PMID: 31961992
- PMCID: PMC7154633
- DOI: 10.1002/anie.201915870
Enantioselective Synthesis of 1,12-Disubstituted [4]Helicenes
Abstract
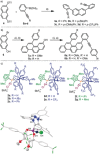
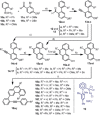
A highly enantioselective synthesis of 1,12-disubstituted [4]carbohelicenes is reported. The key step for the developed synthetic route is a Au-catalyzed intramolecular alkyne hydroarylation, which is achieved with good to excellent regio- and enantioselectivity by employing TADDOL-derived (TADDOL=α,α,α,α-tetraaryl-1,3-dioxolane-4,5-dimethanol) α-cationic phosphonites as ancillary ligands. Moreover, an appropriate design of the substrate makes the assembly of [4]helicenes of different substitution patterns possible, thus demonstrating the synthetic utility of the method. The absolute stereochemistry of the newly prepared structures was determined by X-ray crystallography and characterization of their photophysical properties is also reported.
Keywords: Au catalysis; [4]helicenes; asymmetric catalysis; enantioselective synthesis; ligand design.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

