Enhancing Terminal Deoxynucleotidyl Transferase Activity on Substrates with 3' Terminal Structures for Enzymatic De Novo DNA Synthesis
- PMID: 31963235
- PMCID: PMC7016565
- DOI: 10.3390/genes11010102
Enhancing Terminal Deoxynucleotidyl Transferase Activity on Substrates with 3' Terminal Structures for Enzymatic De Novo DNA Synthesis
Abstract
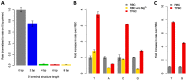
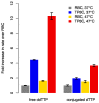
Enzymatic oligonucleotide synthesis methods based on the template-independent polymerase terminal deoxynucleotidyl transferase (TdT) promise to enable the de novo synthesis of long oligonucleotides under mild, aqueous conditions. Intermediates with a 3' terminal structure (hairpins) will inevitably arise during synthesis, but TdT has poor activity on these structured substrates, limiting its usefulness for oligonucleotide synthesis. Here, we described two parallel efforts to improve the activity of TdT on hairpins: (1) optimization of the concentrations of the divalent cation cofactors and (2) engineering TdT for enhanced thermostability, enabling reactions at elevated temperatures. By combining both of these improvements, we obtained a ~10-fold increase in the elongation rate of a guanine-cytosine hairpin.
Keywords: DNA data storage; TdT; enzymatic DNA synthesis; oligonucleotide synthesis; polymerase cofactors; secondary structures; template-independent polymerase; terminal deoxynucleotidyl transferase; thermostability engineering.
Conflict of interest statement
S.P. and D.H.A. are co-founders of Ansa Biotechnologies, Inc., a company commercializing the polymerase-nucleotide conjugate technology. S.P., D.H.A., and S.B. are currently employed by Ansa Biotechnologies, and all authors have a financial interest in Ansa Biotechnologies. J.D.K. and N.J.H. have a financial interest in Ansa Biotechnologies.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

