SV40 Transfected Human Anterior Cruciate Ligament Derived Ligamentocytes-Suitable as a Human in Vitro Model for Ligament Reconstruction?
- PMID: 31963350
- PMCID: PMC7014138
- DOI: 10.3390/ijms21020593
SV40 Transfected Human Anterior Cruciate Ligament Derived Ligamentocytes-Suitable as a Human in Vitro Model for Ligament Reconstruction?
Abstract
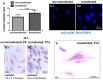
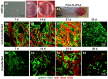
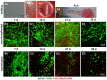
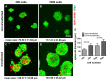
Cultured human primary cells have a limited lifespan undergoing dedifferentiation or senescence. Anterior cruciate ligaments (ACL) are hypocellular but tissue engineering (TE) requires high cell numbers. Simian virus (SV) 40 tumor (T) antigen expression could extend the lifespan of cells. This study aimed to identify cellular changes induced by SV40 expression in human ACL ligamentocytes by comparing them with non-transfected ligamentocytes and tissue of the same donor to assess their applicability as TE model. Human ACL ligamentocytes (40-year-old female donor after ACL rupture) were either transfected with a SV40 plasmid or remained non-transfected (control) before monitored for SV40 expression, survival, and DNA content. Protein expression of cultured ligamentocytes was compared with the donor tissue. Ligamentocyte spheroids were seeded on scaffolds embroidered either from polylactic acid (PLA) threads solely or combined PLA and poly (L-lactide-co-ε-caprolactone) (P(LA-CL)) threads. These scaffolds were further functionalized with fluorination and fibrillated collagen foam. Cell distribution and survival were monitored for up to five weeks. The transfected cells expressed the SV40 antigen throughout the entire observation time, but often exhibited random and incomplete cell divisions with significantly more dying cells, significantly more DNA and more numerous nucleoli than controls. The expression profile of non-transfected and SV40-positive ligamentocytes was similar. In contrast to controls, SV40-positive cells formed larger spheroids, produced less vimentin and focal adhesions and died on the scaffolds after 21 d. Functionalized scaffolds supported human ligamentocyte growth. SV40 antigen expressing ligamentocytes share many properties with their non-transfected counterparts suggesting them as a model, however, applicability for TE is limited.
Keywords: P(LA-CL); PLA; SV40; anterior cruciate ligament; embroidered scaffold; ligamentocytes; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Kudo Y., Hiraoka M., Kitagawa S., Miyauchi M., Kakuo S., Zhao M., Ide T., Takata T. Establishment of human cementifying fibroma cell lines by transfection with temperature-sensitive simian virus-40 T-antigen gene and hTERT gene. Bone. 2002;30:712–717. doi: 10.1016/S8756-3282(02)00689-0. - DOI - PubMed
-
- McNutt N.S., Culp L.A., Black P.H. Contact-inhibited revertant cell lines isolated from SV 40-transformed cells. IV. Microfilament distribution and cell shape in untransformed, transformed, and revertant Balb-c 3T3 cells. J. Cell Biol. 1973;56:412–428. doi: 10.1083/jcb.56.2.412. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

