Bone Marrow Stromal Cells-Induced Drug Resistance in Multiple Myeloma
- PMID: 31963513
- PMCID: PMC7013615
- DOI: 10.3390/ijms21020613
Bone Marrow Stromal Cells-Induced Drug Resistance in Multiple Myeloma
Abstract
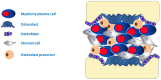
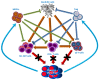
Multiple myeloma is a B-cell lineage cancer in which neoplastic plasma cells expand in the bone marrow and pathophysiological interactions with components of microenvironment influence many biological aspects of the malignant phenotype, including apoptosis, survival, proliferation, and invasion. Despite the therapeutic progress achieved in the last two decades with the introduction of a more effective and safe new class of drugs (i.e., immunomodulators, proteasome inhibitors, monoclonal antibodies), there is improvement in patient survival, and multiple myeloma (MM) remains a non-curable disease. The bone marrow microenvironment is a complex structure composed of cells, extracellular matrix (ECM) proteins, and cytokines, in which tumor plasma cells home and expand. The role of the bone marrow (BM) microenvironment is fundamental during MM disease progression because modification induced by tumor plasma cells is crucial for composing a "permissive" environment that supports MM plasma cells proliferation, migration, survival, and drug resistance. The "activated phenotype" of the microenvironment of multiple myeloma is functional to plasma cell proliferation and spreading and to plasma cell drug resistance. Plasma cell drug resistance induced by bone marrow stromal cells is mediated by stress-managing pathways, autophagy, transcriptional rewiring, and non-coding RNAs dysregulation. These processes represent novel targets for the ever-increasing anti-MM therapeutic armamentarium.
Keywords: drug-resistance; microenvironment; multiple myeloma; plasma cells; stromal cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kumar S.K., Dispenzieri A., Lacy M.Q., Gertz M.A., Buadi F.K., Pandey S., Kapoor P., Dingli D., Hayman S.R., Leung N., et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–1128. doi: 10.1038/leu.2013.313. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

