Elevated Expression of Lumican in Lung Cancer Cells Promotes Bone Metastasis through an Autocrine Regulatory Mechanism
- PMID: 31963522
- PMCID: PMC7016828
- DOI: 10.3390/cancers12010233
Elevated Expression of Lumican in Lung Cancer Cells Promotes Bone Metastasis through an Autocrine Regulatory Mechanism
Abstract
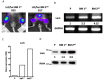
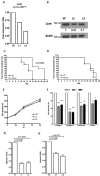
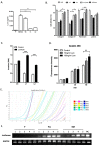
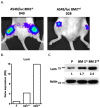
Background: The microarray analysis of whole-genome expression indicated that the gene encoding the protein lumican, which is associated with extracellular matrix (ECM) interaction, was highly expressed in osteotropic lung cancer cell lines with an enhanced capacity of bone metastasis. Methods: The expression of lumican in the osteotropic lung cancer cells was downregulated, and the in vitro migration, invasion, and adhesion of cancer cells to ECM components, and the in vivo bone metastasis capacity of these cells were examined. Exogenous lumican was provided to study the autocrine regulation mechanism of lumican in the bone metastasis of lung cancer cells. Results: Transfection with lumican-specific short hairpin RNA (shRNA) in the osteotropic lung cancer cells reduced the establishment of in vivo bone metastasis, but not lung metastasis. Reduction in the expression of lumican also decreased the attachment of lung osteotropic cancer cells to several components of the ECM and suppressed cell migration and invasion in vitro. Exogenous lumican restored these reduced capacities of lumican knockdown cells and promoted the seeding of lung cancer cells in the bone microenvironment. Conclusions: These results suggested that lumican promotes the metastasis of lung cancer cells to the bones via an autocrine regulatory mechanism, and blocking this interaction may provide a new therapeutic approach to reduce bone metastasis in cases of lung cancer.
Keywords: bone metastasis; lumican; lung cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

