Inhibitory Activity of a Scorpion Defensin BmKDfsin3 against Hepatitis C Virus
- PMID: 31963532
- PMCID: PMC7168052
- DOI: 10.3390/antibiotics9010033
Inhibitory Activity of a Scorpion Defensin BmKDfsin3 against Hepatitis C Virus
Abstract
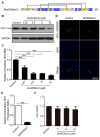
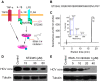
Hepatitis C virus (HCV) infection is a major worldwide health problem which can cause chronic hepatitis, liver fibrosis and hepatocellular carcinoma (HCC). There is still no vaccine to prevent HCV infection. Currently, the clinical treatment of HCV infection mainly relies on the use of direct-acting antivirals (DAAs) which are expensive and have side effects. Here, BmKDfsin3, a scorpion defensin from the venom of Mesobuthus martensii Karsch, is found to dose-dependently inhibit HCV infection at noncytotoxic concentrations and affect viral attachment and post-entry in HCV life cycle. Further experimental results show that BmKDfsin3 not only suppresses p38 mitogen-activated protein kinase (MAPK) activation of HCV-infected Huh7.5.1 cells, but also inhibits p38 activation of Huh7.5.1 cells stimulated by tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) or lipopolysaccharide (LPS). BmKDfsin3 is also revealed to enter into cells. Using an upstream MyD88 dimerization inhibitor ST2345 or kinase IRAK-1/4 inhibitor I, the inhibition of p38 activation represses HCV replication in vitro. Taken together, a scorpion defensin BmKDfsin3 inhibits HCV replication, related to regulated p38 MAPK activation.
Keywords: Hepatitis C virus (HCV); p38; scorpion defensin.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Lei R., Hou J., Chen Q., Yuan W., Cheng B., Sun Y., Jin Y., Ge L., Ben-Sasson S.A., Chen J., et al. Self-assembling myristoylated human alpha-defensin 5 as a next-generation nanobiotics potentiates therapeutic efficacy in bacterial infection. ACS Nano. 2018;12:5284–5296. doi: 10.1021/acsnano.7b09109. - DOI - PubMed
LinkOut - more resources
Full Text Sources

