Microtubules in Influenza Virus Entry and Egress
- PMID: 31963544
- PMCID: PMC7020094
- DOI: 10.3390/v12010117
Microtubules in Influenza Virus Entry and Egress
Abstract
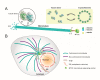
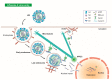
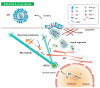
Influenza viruses are respiratory pathogens that represent a significant threat to public health, despite the large-scale implementation of vaccination programs. It is necessary to understand the detailed and complex interactions between influenza virus and its host cells in order to identify successful strategies for therapeutic intervention. During viral entry, the cellular microenvironment presents invading pathogens with a series of obstacles that must be overcome to infect permissive cells. Influenza hijacks numerous host cell proteins and associated biological pathways during its journey into the cell, responding to environmental cues in order to successfully replicate. The cellular cytoskeleton and its constituent microtubules represent a heavily exploited network during viral infection. Cytoskeletal filaments provide a dynamic scaffold for subcellular viral trafficking, as well as virus-host interactions with cellular machineries that are essential for efficient uncoating, replication, and egress. In addition, influenza virus infection results in structural changes in the microtubule network, which itself has consequences for viral replication. Microtubules, their functional roles in normal cell biology, and their exploitation by influenza viruses will be the focus of this review.
Keywords: aggresome processing; cytoskeleton; endocytosis; histone deacetylase; infection biology; influenza virus; microtubules; uncoating.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

