Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model Systems to Estimate Drug Permeability Using an In Vitro Transport Study to Predict Nasal Drug Absorption in Rats
- PMID: 31963555
- PMCID: PMC7023391
- DOI: 10.3390/pharmaceutics12010079
Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model Systems to Estimate Drug Permeability Using an In Vitro Transport Study to Predict Nasal Drug Absorption in Rats
Abstract
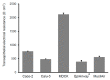
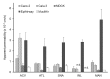
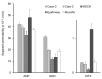
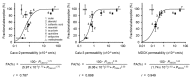
Recently, various types of cultured cells have been used to research the mechanisms of transport and metabolism of drugs. Although many studies using cultured cell systems have been published, a comparison of different cultured cell systems has never been reported. In this study, Caco-2, Calu-3, Madin-Darby canine kidney (MDCK), EpiAirway and MucilAir were used as popular in vitro cell culture systems, and the permeability of model compounds across these cell systems was evaluated to compare barrier characteristics and to clarify their usefulness as an estimation system for nasal drug absorption in rats. MDCK unexpectedly showed the best correlation (r = 0.949) with the fractional absorption (Fn) in rats. Secondly, a high correlation was observed in Calu-3 (r = 0.898). Also, Caco-2 (r = 0.787) and MucilAir (r = 0.750) showed a relatively good correlation with Fn. The correlation between Fn and permeability to EpiAirway was the poorest (r = 0.550). Because EpiAirway forms leakier tight junctions than other cell culture systems, the paracellular permeability was likely overestimated with this system. On the other hand, because MDCK formed such tight cellular junctions that compounds of paracellular model were less likely permeated, the paracellular permeability could be underestimated. Calu-3, Caco-2 and MucilAir form suitable cellular junctions and barriers, indicating that those cell systems enable the precise estimation of nasal drug absorption.
Keywords: Caco-2; Calu-3; MDCK; apparent permeability coefficient; nasal drug absorption; three-dimensional mucociliary tissue model system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Coulet M., Eeckhoutte C., Larrieu G., Sutra J.F., Alvinerie M., Macé K., Pfeifer A., Zucco F., Stammati A.L., De Angelis I., et al. Evidence for cytochrome P4501A2-mediated protein covalent binding of thiabendazole and for its passive intestinal transport: Use of human and rabbit derived cells. Chem. Biol. Interact. 2000;127:109–124. doi: 10.1016/S0009-2797(00)00167-8. - DOI - PubMed
-
- Kato K., Shirasaka Y., Kuraoka E., Kikuchi A., Iguchi M., Suzuki H., Shibasaki S., Kurosawa T., Tamai I. Intestinal absorption mechanism of tebipenem pivoxil, a novel oral carbapenem: Involvement of human OATP family in apical membrane transport. Mol. Pharm. 2010;7:1747–1756. doi: 10.1021/mp100130b. - DOI - PubMed
-
- Kuehn D., Majeed S., Guedj E., Dulize R., Baumer K., Iskandar A., Boue S., Martin F., Kostadinova R., Mathis C., et al. Impact assessment of repeated exposure of organotypic 3D bronchial and nasal tissue culture models to whole cigarette smoke. J. Vis. Exp. 2015;12:52325. doi: 10.3791/52325. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

