Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson's Disease
- PMID: 31963575
- PMCID: PMC7019526
- DOI: 10.3390/jcm9010257
Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson's Disease
Abstract
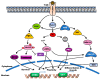
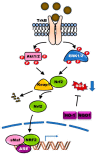
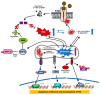
Brain-derived neurotrophic factor (BDNF) and its receptor tropomyosin-related kinase receptor type B (TrkB) are widely distributed in multiple regions of the human brain. Specifically, BDNF/TrkB is highly expressed and activated in the dopaminergic neurons of the substantia nigra and plays a critical role in neurophysiological processes, including neuro-protection and maturation and maintenance of neurons. The activation as well as dysfunction of the BDNF-TrkB pathway are associated with neurodegenerative diseases. The expression of BDNF/TrkB in the substantia nigra is significantly reduced in Parkinson's Disease (PD) patients. This review summarizes recent progress in the understanding of the cellular and molecular roles of BNDF/TrkB signaling and its isoform, TrkB.T1, in Parkinson's disease. We have also discussed the effects of current therapies on BDNF/TrkB signaling in Parkinson's disease patients and the mechanisms underlying the mutation-mediated acquisition of resistance to therapies for Parkinson's disease.
Keywords: BDNF; Parkinson’s disease; TrkB; TrkB isoform; neuronal degeneration.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

