Age-Associated TET2 Mutations: Common Drivers of Myeloid Dysfunction, Cancer and Cardiovascular Disease
- PMID: 31963585
- PMCID: PMC7014315
- DOI: 10.3390/ijms21020626
Age-Associated TET2 Mutations: Common Drivers of Myeloid Dysfunction, Cancer and Cardiovascular Disease
Abstract
Acquired, inactivating mutations in Tet methylcytosine dioxygenase 2 (TET2) are detected in peripheral blood cells of a remarkable 5%-10% of adults greater than 65 years of age. They impart a hematopoietic stem cell advantage and resultant clonal hematopoiesis of indeterminate potential (CHIP) with skewed myelomonocytic differentiation. CHIP is associated with an overall increased risk of transformation to a hematological malignancy, especially myeloproliferative and myelodysplastic neoplasms (MPN, MDS) and acute myeloid leukemia (AML), of approximately 0.5% to 1% per year. However, it is becoming increasingly possible to identify individuals at greatest risk, based on CHIP mutational characteristics. CHIP, and particularly TET2-mutant CHIP, is also a novel, significant risk factor for cardiovascular diseases, related in part to hyper-inflammatory, progeny macrophages carrying TET2 mutations. Therefore, somatic TET2 mutations contribute to myeloid expansion and innate immune dysregulation with age and contribute to prevalent diseases in the developed world-cancer and cardiovascular disease. Herein, we describe the impact of detecting TET2 mutations in the clinical setting. We also present the rationale and promise for targeting TET2-mutant and other CHIP clones, and their inflammatory environment, as potential means of lessening risk of myeloid cancer development and dampening CHIP-comorbid inflammatory diseases.
Keywords: NGS; TET2; aging; cancer progression; clinical detection; clonal hematopoiesis; comorbid disease; driver mutations; inflammation; targeting TET2 therapeutically.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

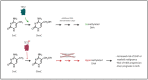
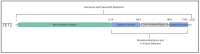
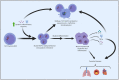
References
-
- Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2406. doi: 10.1182/blood-2016-03-643544. - DOI - PubMed
-
- Barbui T., Thiele J., Gisslinger H., Kvasnicka H.M., Vannucchi A.M., Guglielmelli P., Orazi A., Tefferi A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: Document summary and in-depth discussion. Blood Cancer J. 2018;8:15. doi: 10.1038/s41408-018-0054-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

